Abstract
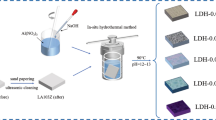
In situ-grown Mg–Al layered double hydroxide (LDH) films were obtained on an anodized AZ31 substrate, with the immersion of sample in different concentrations of Al3+ solution. The structure, composition and morphology of LDH films were investigated by X-ray diffraction (XRD), Fourier transform infrared (FTIR) and scanning electronic microscopy (SEM), and the corrosion behavior of LDH films was further studied by electrochemical impedance spectroscopy (EIS). The influence of Al3+ concentration on the growth behavior of LDH was also discussed. The results indicated that the nest-like structure of MgAl-LDH film was composed of interconnected MgAl-LDH nanosheets. Besides, the LDH obtained in 0.032 mol·L−1 Al3+ solution, possessing dense laminated structure, could effectively seal the porous surface of anodic oxide film. EIS results revealed that the samples coated with LDH films showed a higher electrochemical impedance, and thus, the corrosion resistance of samples coated with LDH films was signally improved compared with the anodized alloy.







Similar content being viewed by others
References
Wang XJ, Xu DK, Wu RZ, Chen XB, Peng QM, Jin L, Xin YC, Zhang ZQ, Liu Y, Chen XH, Chen G, Deng KK, Wang HY. What is going on in magnesium alloys? J Mater Sci Technol. 2018;34(2):245.
Feng B, Xin YC, Guo FL, Yu HH, Wu Y, Liu Q. Compressive mechanical behavior of Al/Mg composite rods with different types of Al sleeve. Acta Mater. 2016;120(8):379.
Gao SY, Chen XH, Pan FS, Song K, Zhao CY, Liu LZ, Liu XF, Zhao FS. Effect of secondary phase on the electromagnetic shielding effectiveness of magnesium alloy. Sci Rep. 2018;8(1):1625.
Wang BJ, Xu DK, Wang SD, Sheng LY, Zeng RC, Han EH. Influence of solution treatment on the corrosion fatigue behavior of an as-forged Mg-Zn-Y-Zr alloy. Int J Fatigue. 2019;120:46.
Liu QL, Wang XD, Wang XK, Yan XW, Ma XJ. Study of high emittance chemical conversion coatings for magnesium alloys. Surf Eng. 2014;30(1):48.
Adsul SH, Raju KRCS, Sarada BV, Sonawane SH, Subasri R. Evaluation of self-healing properties of inhibitor loaded nanoclay-based anticorrosive coatings on magnesium alloy AZ91D. J Magnes Alloys. 2018;6(3):299.
Hu FP, Qin J, Zeng QW, Wang F, Zhang X, Zhang NY, Wang SZ, Huang P, Fan ZH, Peng XD, Xie WD. Research on surface treatment of magnesium alloy and its wettability with resin. Mater Sci Technol. 2016;32(10):963.
Song GL, Atrens A. Corrosion mechanisms of magnesium alloys. Adv Eng Mater. 1999;1(1):11.
Zhang G, Wu L, Tang AT, Ma YL, Song GL, Zheng DJ, Jiang B, Atrens A, Pan FS. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros Sci. 2018;139(5):370.
Lu XP, Blawert C, Tolnai D, Subroto T, Kainer KU, Zhang T, Wang FH, Zheludkevich ML. 3D reconstruction of plasma electrolytic oxidation coatings on Mg alloy via synchrotron radiation tomography. Corros Sci. 2018;139(7):395.
Wu L, Pan FS, Liu YH, Zhang G, Tang AT, Atrens A. Influence of pH on the growth behaviour of Mg–Al LDH films. Surf Eng. 2018;34(9):674.
Gusieva K, Davies CHJ, Scully JR, Birbilis N. Corrosion of magnesium alloys: the role of alloying. Int Mater Rev. 2015;60(3):169.
Zhang F, Zhang CL, Song L, Zeng RC, Liu ZG, Cui HZ. Corrosion of in situ grown MgAl-LDH coating on aluminum alloy. Trans Nonferr Met Soc China. 2015;25(10):3498.
Wang Y, Zhang D, Lu Z. Hydrophobic Mg–Al layered double hydroxide film on aluminum: fabrication and microbiologically influenced corrosion resistance properties. Colloids Surf A. 2015;474(6):44.
Wu FX, Liang J, Peng ZJ, Liu BX. Electrochemical deposition and characterization of Zn–Al layered double hydroxides (LDHs) films on magnesium alloy. Appl Surf Sci. 2014;313(9):834.
Zhang Y, Liu JH, Li YL, Yu M, Li SM, Xue B. A facile approach to superhydrophobic LiAl-layered double hydroxide film on Al–Li alloy substrate. J Coat Technol Res. 2015;12(3):595.
Zhang G, Wu L, Tang AT, Weng B, Atrens A, Ma SD, Liu L, Pan FS. Sealing of anodized magnesium alloy AZ31 with MgAl layered double hydroxides layers. RSC Adv. 2018;4(8):2248.
Ishizaki T, Kamiyama N, Watanabe K, Serizawa A. Corrosion resistance of Mg(OH)2/Mg–Al layered double hydroxide composite film formed directly on combustion-resistant magnesium alloy AMCa602 by steam coating. Corros Sci. 2015;92(3):76.
Wang Y, Zhang D, Lv DD, Sun Y. Mg–Al mixed metal oxide film derived from layered double hydroxide precursor film: fabrication and antibacterial properties. J Taiwan Inst Eng. 2015;57(12):160.
Wu L, Zhang G, Tang AT, Liu YL, Atrens A, Pan FS. Communication–fabrication of protective layered double hydroxide films by conversion of anodic films on magnesium alloy. J Electrochem Soc. 2017;164(7):C339.
Zhang G, Wu L, Tang AT, Pan HL, Ma YL, Zhan Q, Tan QY, Pan FS, Atrens A. Effect of micro-arc oxidation coatings formed at different voltages on the in situ growth of layered double hydroxides and their corrosion protection. J Electrochem Soc. 2018;165(7):C317.
Chen YX, Shui ZH, Chen W, Li Q, Chen GW. Effect of MgO content of synthetic slag on the formation of Mg–Al LDHs and sulfate resistance of slag-fly ash-clinker binder. Constr Build Mater. 2016;125(10):776.
Liu W, Li MC, Luo Q. Influence of alloyed magnesium on the microstructure and long-term corrosion behavior of hot-dip Al–Zn–Si coating in NaCl solution. Corros Sci. 2016;104(3):217.
Wu L, Yang DN, Zhang G, Zhang Z, Zhang S, Tang AT, Pan FS. Fabrication and characterization of Mg–M layered double hydroxide films on anodized magnesium alloy AZ31. Appl Surf Sci. 2018;431(2):177.
Wu L, Zheng ZC, Pan FS, Tang AT, Zhang G, Liu L. Influence of reaction temperature on the controlled growth of Mg–Al LDH film. Int J Electrochem Sci. 2017;12(7):6352.
Qu J, Zhang QW, Li XW, He XM, Song SX. Mechanochemical approaches to synthesize layered double hydroxides: a review. Appl Clay Sci. 2016;119(2):185.
Prosek T, Persson D, Stoulil J, Thierry D. Composition of corrosion products formed on Zn–Mg, Zn–Al and Zn–Al–Mg coatings in model atmospheric conditions. Corros Sci. 2014;86(9):231.
Yeganeh M, Mohammadi N. Superhydrophobic surface of Mg alloys: A review. J Magnes Alloys. 2018; 6(1):59.
Azevedo MS, Allély C, Ogle K, Volovitch P. Corrosion mechanisms of Zn(Mg, Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros Sci. 2015;90(7):482.
Song GL, Atrens A, John DST, Wu X, Nairn J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corros Sci. 1997;39(5):1981.
Cai J, Heng HM, Hu XP, Xu QK, Miao F. A facile method for the preparation of novel fire-retardant layered double hydroxide and its application as nanofiller in UP. Polym Degr Stab. 2016;126(4):47.
Alibakhshi E, Ghasemi E, Mahdavian M, Ramezanzadeh B. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Corros Sci. 2017; 115(2):159.
Diler E, Rouvellou B, Rioual S, Lescop B, Vien GN, Thierry D. Characterization of corrosion products of Zn and Zn–Mg–Al coated steel, in a marine atmosphere. Corros Sci. 2014;87(10):111.
Tedim J, Kuznetsova A, Salak AN, Montemor F, Snihirova D, Pilz M, Zheludkevich ML, Ferreira MGS. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros Sci. 2012; 55(2):1.
Azevedo MS, Allély C, Ogle K. Corrosion mechanisms of Zn(Mg, Al) coated steel in accelerated tests and natural exposure: 1. The role of electrolyte composition in the nature of corrosion products and relative corrosion rate. Corros Sci. 2015;90(1):472.
Chen J, Song YW, Shan DY, Han EH. Study of the in situ growth mechanism of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros Sci. 2012;63(10):148.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFB0301100), the National Natural Science Foundation of China (51701029, 51531002, 51474043), China Postdoctoral Science Foundation Funded Project (2017M620410, 2018T110942), the Chongqing Postdoctoral Scientific Research Foundation (Xm2017010) and the Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0388, cstc2017jcyjBX0040).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, L., Ding, XX., Zhao, XF. et al. Morphology, structure and corrosion resistance of Mg–Al LDH films fabricated in different Al3+ concentration solutions. Rare Met. 42, 697–704 (2023). https://doi.org/10.1007/s12598-018-1191-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-018-1191-y