Abstract
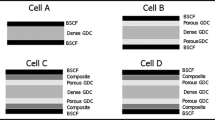
Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) is one of the most active cathode materials and shows significant hydration effect suggesting possible proton conductivity. In this study, the performance of BSCF cathode on a proton-conducting BaZr0.1Ce0.7Y0.2O3−δ (BZCY) electrolyte with silver and gold current collectors was determined. The electrochemical characteristics of the symmetrical and anode-supported cell with diluted silver electrode, silver current collector or gold current collector on BSCF electrode were compared. The significant result is that, although the diluted silver electrode itself shows poor operation stability, the silver current collector has strong electrocatalytic contribution to the BSCF cathode performance on the proton-conducting electrolyte, leading to higher cell performance than that with the gold current collector.







Similar content being viewed by others
References
Steele BCH, Heinzel A. Materials for fuel-cell technologies. Nature. 2001;414(6861):345.
Jiang SP, Chan SH. A review of anode materials development in solid oxide fuel cells. J Mater Sci. 2004;39(14):4405.
Hibino T, Hashimoto A, Suzuki M, Sano M. A solid oxide fuel cell using Y-doped BaCeO3 with Pd-loaded FeO anode and Ba0.5Pr0.5CoO3 cathode at low temperatures. J Electrochem Soc. 2002;149(11):A1503.
Fabbri E, Bi L, Pergolesi D, Traversa E. Towards the next generation of solid oxide fuel cells operating below 600 degrees C with chemically stable proton-conducting electrolytes. Adv Mater. 2012;24(2):195.
He F, Wu TZ, Peng RR, Xia CR. Cathode reaction models and performance analysis of Sm0.5Sr0.5CoO3−δ -BaCe0.8Sm0.2O3−δ composite cathode for solid oxide fuel cells with proton conducting electrolyte. J Power Sources. 2009;194(1):263.
Peng RR, Wu TZ, Liu W, Liu XQ, Meng GY. Cathode processes and materials for solid oxide fuel cells with proton conductors as electrolytes. J Mater Chem. 2010;20(30):6218.
Shao Q, Ge WJ, Lu X, Chen Y, Ding Y, Lin B, Ling Y. A promising cathode for proton-conducting intermediate temperature solid oxide fuel cells: Y0.8Ca0.2BaCo4O7+δ . Ceram Int. 2015;41(5):6687.
Wang MS, Wang JX, He CR, Xue YJ, Miao H, Wang Q, Wang WG. A novel composite cathode La0.6Sr0.4CoO3−δ - BaZr0.1Ce0.7Y0.1Yb0.1O3−δ for intermediate temperature solid oxide fuel cells. Ceram Int. 2015;41(3):5017.
Mao X, Wang W, Ma G. A novel cobalt-free double-perovskite NdBaFe1.9Nb0.1O5+δ cathode material for proton-conducting IT-SOFC. Ceram Int. 2015;41(8):10276.
Shao ZP, Haile SM. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature. 2004;431(7005):170.
Jeon SY, Lim DK, Kim IH, Singh B, Song SJ. Effectiveness of protonic conduction in Ba0.5Sr0.5Co0.8Fe0.2O3−δ cathode in intermediate temperature proton-conducting ceramic-electrolyte fuel cell. J Electrochem Soc. 2014;161(6):F754.
Lim DK, Singh B, Choi MB, Song SJ. Study of hydration/dehydration kinetics of SOFC cathode material Ba0.5Sr0.5Co0.8Fe0.2O3-δ by electrical conductivity relaxation technique. J Electrochem Soc. 2013;160(8):F764.
Simner S, Anderson M, Pederson L, Stevenson J. Performance variability of La (Sr) FeO3 SOFC cathode with Pt, Ag, and Au current collectors. J Electrochem Soc. 2005;152(9):A1851.
De Silva K, Kaseman B, Bayless D. Silver (Ag) as anode and cathode current collectors in high temperature planar solid oxide fuel cells. Int J Hydrogen Energy. 2011;36(1):779.
Guo YM, Liu Y, Cai R, Chen DJ, Ran R, Shao ZP. Electrochemical contribution of silver current collector to oxygen reduction reaction over Ba0.5Sr0.5Co0.8Fe0.2O3−δ electrode on oxygen-ionic conducting electrolyte. Int J Hydrogen Energy. 2012;37(19):14492.
Jiang SP, Love J, Apateanu L. Effect of contact between electrode and current collector on the performance of solid oxide fuel cells. Solid State Ion. 2003;160(1–2):15.
Guo YM, Zhou YB, Chen DJ, Shi HG, Ran R, Shao ZP. Significant impact of the current collection material and method on the performance of Ba0.5Sr0.5Co0.8Fe0.2O3−δ electrodes in solid oxide fuel cells. J Power Sources. 2011;196(13):5511.
Chen YB, Wang FC, Chen DJ, Dong FF, Park HJ, Kwak C, Shao ZP. Role of silver current collector on the operational stability of selected cobalt-containing oxide electrodes for oxygen reduction reaction. J Power Sources. 2012;210:146.
Adanuvor PK, White RE. Oxygen reduction on silver in 6.5 M caustic soda solution. J Electrochem Soc. 1988;135(10):2509.
Kongkanand A, Kuwabata S. Oxygen reduction at silver monolayer islands deposited on gold substrate. Electrochem Commun. 2003;5(2):133.
Egger A, Sitte W. Enhanced oxygen surface exchange of La2NiO4+δ by means of a thin surface layer of silver. Solid State Ion. 2014;258:30.
Shao ZP, Zhou W, Zhu ZH. Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Prog Mater Sci. 2012;57(4):804.
Yamaura H, Ikuta T, Yahiro H, Okada G. Cathodic polarization of strontium-doped lanthanum ferrite in proton-conducting solid oxide fuel cell. Solid State Ion. 2005;176(3–4):269.
Lin Y, Ran R, Zheng Y, Shao ZP, Jin WQ, Xu NP, Ahn J. Evaluation of Ba0.5Sr0.5Co0.8Fe0.2O3-delta as a potential cathode for an anode-supported proton-conducting solid-oxide fuel cell. J Power Sources. 2008;180(1):15.
Yang L, Zuo CD, Wang SZ, Cheng Z, Liu ML. A novel composite cathode for low-temperature SOFCs based on oxide proton conductors. Adv Mater. 2008;20(17):3280.
Guo YM, Lin Y, Ran R, Shao ZP. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8−y Y0.2O3−δ (0.0 ≤ y ≤ 0.8) for fuel cell applications. J Power Sources. 2009;193(2):400.
Ma QL, Peng RR, Lin YJ, Gao JF, Meng GY. A high-performance ammonia-fueled solid oxide fuel cell. J Power Sources. 2006;161(1):95.
Peng RR, Wu Y, Yang LZ, Mao ZQ. Electrochemical properties of intermediate-temperature SOFCs based on proton conducting Sm-doped BaCeO3 electrolyte thin film. Solid State Ion. 2006;177(3–4):389.
Lin Y, Ran R, Zheng Y, Shao ZP, Jin WQ, Xu NP, Ahn J. Evaluation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ as a potential cathode for an anode-supported proton-conducting solid-oxide fuel cell. J Power Sources. 2008;180(1):15.
Lin Y, Ran R, Chen DJ, Shao ZP. A novel Ba0.6Sr0.4Co0.9Nb0.1O3−δ cathode for protonic solid-oxide fuel cells. J Power Sources. 2010;195(15):4700.
Ling YH, Yu J, Lin B, Zhang XZ, Zhao L, Liu XQ. A cobalt-free Sm0.5Sr0.5Fe0.8Cu0.2O3−δ -Ce0.8Sm0.2O2−δ composite cathode for proton-conducting solid oxide fuel cells. J Power Sources. 2011;196(5):2631.
Ding HP, Xie YY, Xue XJ. Electrochemical performance of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte based proton-conducting SOFC solid oxide fuel cell with layered perovskite PrBaCo2O5+δ cathode. J Power Sources. 2011;196(5):2602.
Lin Y, Zhou W, Sunarso J, Ran R, Shao ZP. Characterization and evaluation of BaCo0.7Fe0.2Nb0.1O3−δ as a cathode for proton-conducting solid oxide fuel cells. Int J Hydrogen Energy. 2012;37(1):484.
Li ML, Ni M, Su F, Xia CR. Proton conducting intermediate-temperature solid oxide fuel cells using new perovskite type cathodes. J Power Sources. 2014;260:197.
Hou J, Zhu ZW, Qian J, Liu W. A new cobalt-free proton-blocking composite cathode La2NiO4+δ –LaNi0.6Fe0.4O3−δ for BaZr0.1Ce0.7Y0.2O3−δ -based solid oxide fuel cells. J Power Sources. 2014;264:67.
Wang ZQ, Yang WQ, Shafi SP, Bi L, Wang ZB, Peng RR, Xia CR, Liu W, Lu YL. A high performance cathode for proton conducting solid oxide fuel cells. J Mater Chem A. 2015;3(16):8405.
West M, Manthiram A. Synthesis of 3-dimensional silver networks and their application in solid oxide fuel cells. Int J Hydrogen Energy. 2015;40(11):4234.
Adler S. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem Rev. 2004;104(10):4791.
Lin Y, Ran R, Shao ZP. Silver-modified Ba0.5Sr0.5Co0.8Fe0.2O3−δ as cathodes for a proton conducting solid-oxide fuel cell. Int J Hydrogen Energy. 2010;35(15):8281.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51502001), the Natural Science Fund of Anhui Province (No. 1608085MB31), the Provincial Natural Science Research Program of Higher Education Institutions of Anhui Province (No. KJ2015A0501), Anhui University Personnel Recruiting Project of Academic and Technical Leaders (No. J10117700069) and the State Key Laboratory of Materials-Oriented Chemical Engineering (No. KL15-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan, TT., Zhu, AK., Li, HB. et al. Performance variability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ cathode on proton-conducting electrolyte SOFCs with Ag and Au current collectors. Rare Met. 37, 633–641 (2018). https://doi.org/10.1007/s12598-017-0942-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-017-0942-5