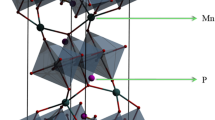
Abstract
LiMnxFe1−xPO4-C cathode materials for lithium ion batteries were synthesized via solid-state method using Li2CO3, MnCO3, NH4H2PO4, FePO4 and sucrose as starting raw materials, followed by high-temperature reduction-annealing. A series of calcination experiments at different temperatures reveal that Mn2+-containing materials exhibit a lower temperature for olivine phase formation, for example LiMn0.5Fe0.5PO4 olivine phase forms at 275 °C, while manganese-free crystalline LiFePO4 generally forms at the required temperature of 350 °C. Increasing Mn2+ content is found to enhance crystallization degree of LiMnxFe1−xPO4 material prepared at lower calcination temperatures. X-ray photoelectron spectroscopy (XPS) results confirm that Mn valence state (+2) remains unchanged up to ~250 °C when calcined in ambient atmosphere. The above-mentioned beneficial effect of manganese on phase formation and crystallization of olivine can be well attributed to the stable nature of Mn2+ and its strong propensity to form olivine phases.







Similar content being viewed by others
References
Zaghib K, Mauger A, Julien CM. Overview of olivines in lithium batteries for green transportation and energy storage. J Solid State Electrochem. 2012;16(3):835.
Liu J, Zhang JG, Yang Z, Lemmon JP, Imhoff C, Graff GL, Li L, Hu J, Wang C, Xiao J, Xia G, Viswanathan VV, Baskaran S, Sprenkle V, Li X, Shao Y, Schwenzer B. Materials science and materials chemistry for large scale electrochemical energy storage: from transportation to electrical grid. Adv Funct Mater. 2013;23(8):929.
Yamada A, Chung SC. Crystal chemistry of the olivine-type LiMnyFe1−yPO4 and MnyFe1−yPO4 as possible 4 V cathode materials for lithium batteries. J Electrochem Soc. 2001;148(8):A960.
Drezen T, Kwon NH, Bowen P, Teerlinck I, Isono M, Exnar I. Effect of particle size on LiMnPO4 cathodes. J Power Sources. 2007;174(2):949.
Goodenough JB, Kim Y. Challenges for rechargeable batteries. J Power Sources. 2011;196(16):6688.
Amine K, Kanno R, Tzeng Y. Rechargeable lithium batteries and beyond: progress, challenges, and future directions. MRS Bull. 2014;39(05):395.
Deng LZ, Wu F, Gao XG, Wu WP. Development of a LiFePO4 based high power lithium secondary battery for HEVs applications. Rare Met. 2014. doi:10.1007/s12598-014-0316-1.
Lu ZG, Tan XX, Tang YG, Zhou KC. LiNi0.7Co0.15Mn0.15O2 microspheres as high-performance cathode materials for lithium–ion batteries. Rare Met. 2014;33(5):608.
Wang JH, Wang Y, Guo YZ, Liu CW, Dan LL. Electrochemical characterization of AlPO4 coated LiNi1/3Co1/3Mn1/3O2 cathode materials for high temperature lithium battery application. Rare Met. 2014. doi:10.1007/s12598-014-0247-x.
Qing C, Bai Y, Yang J, Zhang W. Enhanced cycling stability of LiMn2O4 cathode by amorphous FePO4 coating. Electrochim Acta. 2011;56(19):6612.
Choi D, Wang D, Bae IT, Xiao J, Nie Z, Wang W, Viswanathan VV, Lee YJ, Zhang JG, Graff GL, Yang Z, Liu J. LiMnPO4 nanoplate grown via solid-state reaction in molten hydrocarbon for Li-ion battery cathode. Nano Lett. 2010;10(8):2799.
Morgan D, Van der Ven A, Ceder G. Li conductivity in LixMPO4 (M = Mn, Fe Co, Ni) olivine materials. Electrochem Solid State Lett. 2004;7(2):A30.
Bramnik NN, Bramnik KG, Nikolowski K, Hinterstein M, Baehtz C, Ehrenberg H. Synchrotron diffraction study of lithium extraction from LiMn0.6Fe0.4PO4. Electrochem Solid State Lett. 2005;8(8):A379.
Saravanan K, Vittal JJ, Reddy MV, Chowdari BVR, Balaya P. Storage performance of LiFe1−xMnxPO4 nanoplates (x = 0, 0.5, and 1). J Solid State Electrochem. 2010;14(10):1755.
Aravindan V, Gnanaraj J, Lee YS, Madhavi S. LiMnPO4-A next generation cathode material for lithium–ion batteries. J Mater Chem A. 2013;1(11):3518.
Yamada A, Chung SC, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc. 2001;148(3):A224.
Yamada A, Hosoya M, Chung SC, Kudo Y, Hinokuma K, Liu K-Y, Nishi Y. Olivine-type cathodes: achievements and problems. J Power Sources. 2003;119–121:232.
Martha SK, Grinblat J, Haik O, Zinigrad E, Drezen T, Miners JH, Exnar I, Kay A, Markovsky B, Aurbach D. LiMn0.8Fe0.2PO4: an advanced cathode material for rechargeable lithium batteries. Angew Chem Int Ed. 2009;48(45):8559.
Shiratsuchi T, Okada S, Doi T, Yamaki JI. Cathodic performance of LiMn1−xMxPO4 (M = Ti, Mg and Zr) annealed in an inert atmosphere. Electrochim Acta. 2009;54(11):3415.
Xiao J, Xu W, Choi D, Zhang JG. Synthesis and characterization of lithium manganese phosphate by a precipitation method. J Electrochem Soc. 2010;157(2):A142.
Oh SM, Myung ST, Park JB, Scrosati B, Amine K, Sun YK. Double-structured LiMn0.85Fe0.15PO4 coordinated with LiFePO4 for rechargeable lithium batteries. Angew Chem Int Ed. 2012;51(8):1853.
Ravet N, Gauthier M, Zaghib K, Goodenough JB, Mauger A, Gendron F, Julien C. Mechanism of the Fe3+ reduction at low temperature for LiFePO4 synthesis from a polymeric additive. Chem Mater. 2007;19(10):2595.
Neef C, Jähne C, Meyer HP, Klingeler R. Morphology and agglomeration control of LiMnPO4 micro- and nanocrystals. Langmuir. 2013;29(25):8054.
Jo M, Yoo H, Jung YS, Cho J. Carbon-coated nanoclustered LiMn0.71Fe0.29PO4 cathode for lithium–ion batteries. J Power Sources. 2012;216:162.
Molenda J, Ojczyk W, Swierczek K, Zajac W, Krok F, Dygas J, Liu R. Diffusional mechanism of deintercalation in LiFe1−yMnyPO4 cathode material. Solid State Ionics. 2006;177(26–32):2617.
Oh SM, Sun YK. Improving the electrochemical performance of LiMn0.85Fe0.15PO4–LiFePO4 core-shell materials based on an investigation of carbon source effect. J Power Sources. 2013;244:663.
Wang ZH, Yuan LX, Zhang WX, Huang YH. LiFe0.8Mn0.2PO4/C cathode material with high energy density for lithium–ion batteries. J Alloy Compd. 2012;532:25.
Du K, Zhang LH, Cao YB, Peng ZD, Hu GR. Synthesis of LiMn0.8Fe0.2PO4/C by co-precipitation method and its electrochemical performances as a cathode material for lithium–ion batteries. Mater Chem Phys. 2012;136(2–3):925.
Zhang B, Wang X, Liu Z, Li H, Huang X. Enhanced electrochemical performances of carbon coated mesoporous LiFe0.2Mn0.8PO4. J Electrochem Soc. 2010;157(3):A285.
Cao Y, Duan J, Hu G, Jiang F, Peng Z, Du K, Guo H. Synthesis and electrochemical performance of nanostructured LiMnPO4/C composites as lithium–ion battery cathode by a precipitation technique. Electrochim Acta. 2013;98:183.
Bakenov Z, Taniguchi I. LiMnPO4 olivine as a cathode for lithium batteries. Open Mater Sci J. 2011;5(1):222.
Barpanda P, Djellab K, Recham N, Armand M, Tarascon JM. Direct and modified ionothermal synthesis of LiMnPO4 with tunable morphology for rechargeable Li-ion batteries. J Mater Chem. 2011;21(27):10143.
Kumar PR, Venkateswarlu M, Misra M, Mohanty AK, Satyanarayana N. Carbon coated LiMnPO4 nanorods for lithium batteries. J Electrochem Soc. 2011;158(3):A227.
Kumar PR, Venkateswarlu M, Misra M, Mohanty AK, Satyanarayana N. Enhanced conductivity and electrical relaxation studies of carbon-coated LiMnPO4 nanorods. Ionics. 2012;19(3):461.
Kang J, Song J, Kim S, Gim J, Jo J, Mathew V, Han J, Kim J. A high voltage LiMnPO4–LiMn2O4 nanocomposite cathode synthesized by a one-pot pyro synthesis for Li-ion batteries. RSC Advances. 2013;3(48):25640.
Wang L, Sun W, He X, Li J, Jiang C. Synthesis of nano-LiMnPO4 from MnPO4·H2O prepared by mechanochemistry. Int J Electrochem Sci. 2011;6:2022.
Kwon NH, Fromm KM. Enhanced electrochemical performance of <30 nm thin LiMnPO4 nanorods with a reduced amount of carbon as a cathode for lithium ion batteries. Electrochim Acta. 2012;69:38.
Kwon NH, Drezen T, Exnar I, Teerlinck I, Isono M, Graetzel M. Enhanced electrochemical performance of mesoparticulate LiMnPO4 for lithium ion batteries. Electrochem Solid State Lett. 2006;9(6):A277.
Damen L, De Giorgio F, Monaco S, Veronesi F, Mastragostino M. Synthesis and characterization of carbon-coated LiMnPO4 and LiMn1−xFexPO4 (x = 0.2, 0.3) materials for lithium–ion batteries. J Power Sources. 2012;218:250.
Yoncheva M, Koleva V, Mladenov M, Sendova-Vassileva M, Nikolaeva-Dimitrova M, Stoyanova R, Zhecheva E. Carbon-coated nano-sized LiFe1−xMnxPO4 solid solutions (0 ≤ x ≤ 1) obtained from phosphate-formate precursors. J Mater Sci. 2011;46(22):7082.
Chen J, Vacchio MJ, Wang S, Chernova N, Zavalij PY, Whittingham MS. The hydrothermal synthesis and characterization of olivines and related compounds for electrochemical applications. Solid State Ionics. 2008;178(31–32):31.
Chen L, Yuan YQ, Feng X, Li MW. Enhanced electrochemical properties of LiFe1−xMnxPO4/C composites synthesized from FePO4·2H2O nanocrystallites. J Power Sources. 2012;214:344.
Amisse R, Hamelet S, Hanzel D, Courty M, Dominko R, Masquelier C. Nonstochiometry in LiFe0.5Mn0.5PO4: structural and electrochemical properties. J Electrochem Soc. 2013;160(9):A1446.
Chikkannanavar SB, Bernardi DM, Liu L. A review of blended cathode materials for use in Li-ion batteries. J Power Sources. 2014;248:91.
Jiang J, Kucernak A. Electrochemical supercapacitor material based on manganese oxide: preparation and characterization. Electrochim Acta. 2002;47(15):2381.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 51202014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, GW., Zhang, XJ., Gong, S. et al. Phase formation and crystallization of LiMnxFe1−xPO4-C olivine material with different Mn2+ contents fabricated at lower calcination temperatures. Rare Met. 41, 3142–3149 (2022). https://doi.org/10.1007/s12598-015-0603-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0603-5