Abstract
In Syrian hamsters, brown adipose tissue (BAT) develops postnatally through the proliferation and differentiation of brown adipocyte progenitors. In the study reported here, we investigated how ambient temperature influenced BAT formation in neonatal hamsters. In both hamsters raised at 23 or 30 °C, the interscapular fat changed from white to brown coloration in an age-dependent manner and acquired the typical morphological features of BAT by day 16. However, the expression of uncoupling protein 1, a brown adipocyte marker, and of vascular endothelial growth factor α were lower in the group raised at 30 °C than in that raised at 23 °C. Immunofluorescent staining revealed that the proportion of Ki67-expressing progenitors and endothelial cells was lower in the 30 °C group than in the 23 °C group. These results indicate that warm ambient temperature suppresses the proliferation of brown adipocyte progenitors and endothelial cells and negatively affects the postnatal development of BAT in Syrian hamsters.
Similar content being viewed by others
Introduction
White and brown adipocytes, two types of fat tissue in mammals, have quite different morphologies and physiological roles [1,2,3]. White adipocytes, the major component of white adipose tissue (WAT), contain large unilocular lipid droplets in their cytoplasm and store excess energy as triglycerides. Brown adipocytes in brown adipose tissue (BAT) contain numerous small lipid droplets (multilocular) and consume fatty acids as substrates for non-shivering thermogenesis. The thermogenic activity of BAT is dependent on mitochondrial uncoupling protein 1 (UCP1), which dissipates the proton gradient as heat. The generated heat is transferred throughout the body via the dense vascular system within the BAT [1, 4].
BAT thermogenesis is activated by cold stimulation through the sympathetic nervous system and contributes to the maintenance of body temperature in a cold environment [5, 6]. This thermogenesis is particularly important in newborns, especially at birth and during the early neonatal period, to maintain their body temperature at a rather lower ambient temperature than that in the womb [1]. Thus, most mammals are born with functional BAT expressing UCP1, and the BAT actively starts to produce heat immediately after birth. In this context, Syrian hamsters are unique: newborns do not have typical BAT at birth, but instead develop this tissue during the neonatal period [7, 8]. Recently, we reported that interscapular fat, where BAT is located in most mammals, mainly consists of white adipocytes at birth, but that brown adipocyte progenitors, which appear postnatally within the tissue, proliferate and differentiate into brown adipocytes during the neonatal period [9]. In addition, white adipocytes gradually convert to brown adipocytes to also form BAT. From the perspective of tissue morphology and UCP1 expression, BAT formation is likely completed around postnatal day 16. This time course of BAT formation is consistent with the characteristics of body temperature regulation in neonatal hamsters: they have been reported to behave as poikilotherms, with their body temperature changing depending on the ambient temperature, until approximately 21 days of age [10]. However, the trigger or mechanisms that regulate BAT formation in neonatal hamsters are unknown.
Although most mammals possess mature BAT at birth, the environmental temperature during the neonatal period significantly influences BAT development. In newborn mice, which are precocial-type newborns that show enhanced UCP1 expression during the neonatal period [11], the postnatal increase in UCP1 expression is suppressed in a warm environment [12]. Conversely, in newborn guinea pigs, which are altricial-type newborns that show a peak of UCP1 expression at birth which decreases thereafter, the postnatal decline in UCP1 expression is attenuated in a cold environment [13]. Thus, it is possible that postnatal BAT formation in hamsters is induced by the change in environmental temperature that newborn hamsters experience at the time of birth.
In the study reported here, we investigated the effect of ambient temperature on BAT formation in neonatal hamsters.
Methods
Materials
Antibodies against Ki67 and MCT1 were from Millipore (Billerica, MA, USA) and Abcam (Cambridge, MA, USA), respectively. Anti-rabbit or anti-chicken secondary antibodies, streptavidin conjugated with Alexa Fluor 488 or Alexa Fluor 594 and ProLong Gold Antifade with 4′,6-diamidino-2-phenylindole (DAPI), a fluorescent stain, were obtained from Thermo Fisher Scientific (Gaithersburg, MD, USA). Biotinylated Maackia amurensis agglutinin (MAM) was obtained from J-OIL MILLS, Inc. (Tokyo, Japan).
Animals
The experimental procedures and care of animals were approved by the Animal Care and Use Committee of Hokkaido University (Sapporo, Japan) and conducted in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. Parental Syrian hamsters were purchased from Japan SLC (Shizuoka, Japan) and housed in plastic cages within an air-conditioned room at 23 °C, with a 12:12 h light:dark cycle. These animals were given free access to laboratory chow (Oriental Yeast, Tokyo, Japan) and tap water. Male and female hamsters were mated by putting a female into a cage with a male for 1 week. When pregnancy was confirmed (approximately 10 days after mating), some of the dams were transferred to a room kept at 30 °C. Pups that were born and raised at 23 or at 30 °C were euthanized using carbon dioxide at the indicated day, and their fat tissues from interscapular and inguinal depots were immediately removed and weighed. Tissue specimens were fixed in 10% buffered formalin for histological examination.
Histological analysis
Tissue specimens fixed in 10% buffered formalin were dehydrated through an ethanol series and embedded in paraffin according to the conventional method, cut into 5-µm-thick sections, and stained with hematoxylin and eosin. For immunofluorescent staining, sections were deparaffinized and then incubated with 0.3% hydrogen peroxide in methanol to block endogenous peroxidase. For the detection of Ki67, microwave-enhanced antigen retrieval was performed by boiling the slides in 10 mM citrate buffer (pH 6.0) containing 0.05% Tween 20 for 10 min. After washing in phosphate-buffered saline, the slides were incubated with 10% normal goat serum for 1 h and then with the primary antibodies (rabbit anti-Ki67, 1:200 dilution; or chicken anti-MCT1, 1:200) or biotinylated MAM (1:250) overnight at 4 °C. Slides were washed and incubated with fluorescence-conjugated secondary antibodies or streptavidin (1:200) for 1 h. After washing, the sections were covered with a coverslip with ProLong Gold Antifade and observed under a Keyence microscope (Tokyo, Japan). Photographs taken of the immunostained sections were analyzed in Image J (http://imagej.nih.gov/ij/), and the proportion of area showing positive staining for MAM relative to the whole tissue was measured. The number of cells positively stained with Ki67, MCT1, and MAM, respectively, in four sections per animal was counted by an observer blinded to the treatment.
Real-time PCR
Total RNA (1 µg) was reverse-transcribed using a 15-mer oligo(dT) adaptor primer and M-MLV reverse transcriptase (Thermo Fisher Scientific). PCR was performed using Ex Taq polymerase (Takara Bio Inc., Shiga, Japan) with specific primers as follows: 5′-AAGTGTGACGTCGACATCCG-3′ and 5′-GATCCACACAGAGTACTTGC-3′ for the actin B gene (ActB); 5′-GAGCTGGTAACATATGACCT-3′ and 5′-TGTCCTGGCAGAGAGTTGAT-3′ for the UCP1 gene (Ucp1); 5′-AACAGGAGCACCAATGAGTG-3′ and 5′-TTGGCCTTCATGTCCAGCAT-3′ for the cytochrome oxidase subunit IV gene (Cox4); and 5′-ATCACCATGCAGATCATGCG-3′ and 5′-TTGCAACGCCAGTCTGTGTT-3′ for the vascular endothelial growth factor A gene (Vegfa).
Data analysis
Values are expressed as mean ± standard error. Statistical analyses were performed using Student’s t test or two-way analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test. The P values obtained by two-way ANOVA are shown in Table 1.
Results
To examine the role of ambient temperature in the postnatal development of fat tissues, we raised newborn Syrian hamsters at 23 or 30 °C. The litter sizes were 7.2 ± 0.8 and 8.0 ± 1.1 in the 23 and 30 °C groups, respectively, without any significant difference between groups. The body weight on day 7 did not differ between the 23 and 30 °C groups and had gradually increased by day 25 in both groups (Fig. 1a). The weight of fat tissues from interscapular and inguinal depots also did not differ between the two temperatures on day 7 and showed an age-dependent increase by day 25 (Fig. 1b, c). Interscapular fat weight on day 25 was significantly higher in the 30 °C group than in the 23 °C group.
Effect of ambient temperature on the weight of body and fat tissues in Syrian hamsters. Syrian hamsters were born and bred at 23 °C or 30 °C. On postnatal days 7–25, the body weight (a), and weight of the fat tissue from the interscapular (b) and inguinal (c) depots, respectively, were measured (mean ± standard error [SE], n = 4 per group, two-way analysis of variance [ANOVA] followed by the Tukey–Kramer post hoc test). Asterisk indicates significant difference at P < 0.05 compared to day 7 of the same group; daggar indicates significant different at P < 0.05 between the 23 °C group and 30 °C group. P values obtained by two-way ANOVA are shown in Table 1
Consistent with our previous findings, the color of interscapular fat changed from white at birth to brown during postnatal development, exhibiting a typical BAT appearance by day 16 in the 23 °C group (Fig. 2a). Tissues from the 30 °C group showed a similar white-to-brown change in color by day 14, but no obvious change in color was observed thereafter. Consequently, the color of tissues of the 30 °C group was lighter than that of the tissues of the 23 °C group from days 16 and 25, and similar to that of tissues on day 14 of the 23 °C group.
Effect of ambient temperature on gross and histological appearance of interscapular fat tissue in Syrian hamsters. Interscapular fat tissues were obtained from 7- to 25-day-old Syrian hamsters bred at 23 or 30 °C. a Gross images of interscapular fat. Histological images of interscapular (b) and inguinal (c) fat. Scale bars: a 1 cm; b, c 50 µm (colour figure online)
In the 23 °C group, the interscapular fat of 7-day-old hamsters mainly consisted of white adipocytes containing large unilocular lipid droplets and of a small number of brown adipocyte progenitors scattered within the tissue (Fig. 2b). The area of the progenitors expanded thereafter, filling most of the tissue by day 14 and showing the typical histology of BAT after day 16. The interscapular fat of the 30 °C group showed similar histological changes to that of the 23 °C group, while they had enlarged lipid droplets within each brown adipocyte on days 16 and 25. Inguinal fat tissue, a typical WAT, consisted of white adipocytes irrespective of the postnatal day and its morphology was similar between the 23 °C and 30 °C groups (Fig. 2c).
To examine the effect of ambient temperature on gene expression in interscapular fat, we performed real-time PCR (Fig. 3a). In the 23 °C group, the expression of the brown adipocyte marker Ucp1 increased in a manner dependent on postnatal day in both groups, but it was significantly lower in the 30 °C group than in the 23 °C group on day 25. In contrast, the expression of Cox4, a mitochondrial marker, gradually increased with age in both groups, although there was no difference between the 23 °C and 30 °C groups (Fig. 3b). In accordance with the previous observation that BAT development is accompanied with angiogenesis [9], the expression of Vegfa in the 23 °C group dramatically increased by day 16 and remained high on day 25, suggesting that active vascularization occurred within the tissue during this period. In contrast, Vegfa expression in the 30 °C group increased slightly and transiently by day 16. There were significant differences in this variable between the 23 °C and 30 °C groups on days 16 and 25.
Effect of ambient temperature on gene expression in interscapular fat of Syrian hamsters. Interscapular fat tissues were obtained from 7- to 25-day-old Syrian hamsters bred at 23 or 30 °C. Gene expression of uncoupling protein 1 (Ucp1; a), cytochrome c oxidase subunit IV (Cox4; b), and vascular endothelial growth factor A (Vegfa; c) was measured (mean ± SE, n = 4 per group, two-way ANOVA followed by the Tukey–Kramer post hoc test). Asterisk indicates significant difference at P < 0.05 compared to day 7 of the same group; daggar indicates significant different at P < 0.05 between the 23 °C group and 30 °C group. The P values obtained by two-way ANOVA are shown in Table 1
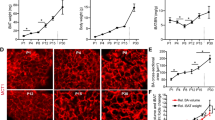
Previously, we reported that brown adipocyte progenitors expressing MCT1 proliferate and differentiate into brown adipocytes during BAT development [9]. Thus, in the current study we examined the effect of ambient temperature on the proliferation of brown adipocyte progenitors. On day 10, cells expressing the proliferation marker Ki67 were detected in the interscapular fat in both groups, and the number of Ki67-positive nuclei did not differ between the 30 °C group (53 ± 2.3% of total number of DAPI-positive nuclei) and the 23 °C group (59 ± 1.1%; Fig. 4a). Dual immunofluorescence staining using antibodies against Ki67 (red) and MCT1 (green) revealed that in the 23 °C group, 23 ± 1.0% of MCT1-positive cells were Ki67-positive-proliferating cells, which was significantly higher than the level in the 30 °C group (11 ± 0.4%) (Fig. 4b).
Effect of ambient temperature on proliferation of brown adipocyte progenitors in interscapular fat of Syrian hamsters. Interscapular fat tissues were obtained from 10-day-old Syrian hamsters bred at 23 °C or 30 °C. a Representative images of the interscapular fat sections stained with antibodies against Ki67 (red) and MCT1 (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar 100 µm. b The proportion of cells which stained double positive for Ki67 and MCT1 antibodies in total MCT1-positive cells was measured (mean ± SE, n = 4 per group, Student’s t test). Asterisk indicates significant difference at P < 0.05 compared to the 23 °C group (colour figure online)
Since Vegfa expression was significantly lower in the 30 °C group than in the 23 °C group (Fig. 3c), we also examined the proliferation of endothelial cells (Fig. 5a). Dual fluorescent staining of interscapular fat of 10-day-old hamsters using antibody against Ki67 and MAM lectin, which binds to the sugar chains that are abundant in vascular endothelial cells, showed that the number of Ki67-positive cells was 13 ± 1.0% of the total MAM-positive cells in the 23 °C group, whereas it was significantly lower in the 30 °C group (8.8 ± 0.3%) (Fig. 5b). These results suggest that angiogenesis was suppressed in the 30 °C group. Consistent with this result, the proportion of MAM-positive area relative to the whole tissue area in interscapular fat of 16-day-old hamsters was significantly lower in the 30 °C group than in the 23 °C group (Fig. 5c, d).
Effect of ambient temperature on the proliferation of endothelial cells in interscapular fat of Syrian hamsters. Interscapular fat tissues were obtained from 10- or 16-day-old Syrian hamsters bred at 23 °C or 30 °C. a Representative images of interscapular fat sections (10-day-old) stained with antibody against Ki67 (red) and Maackia amurensis agglutinin (MAM) lectin (green). Scale bar 100 µm. b The proportion of cells double positive for Ki67 and MAM lectin relative to the total MAM-stained cells was measured. c Representative images of interscapular fat sections (16-day-old) stained with MAM lectin (green). Scale bar 100 µm. d The proportion of MAM-stained area relative to the whole tissue area was measured (mean ± SE, n = 4 per group, Student’s t test). Asterisk indicates significant difference at P < 0.05 compared with the 23 °C group (colour figure online)
Discussion
In this study, we examined the effect of ambient temperature on postnatal BAT development in Syrian hamsters. The warm condition may have exerted some heat stress on the hamsters; however, there was no significant difference in the age-dependent increase in body weight and WAT weight between those hamsters bred in a warm environment (30 °C) and those bred at normal room temperature (23 °C). In addition, the numbers of pups per litter were similar under these two conditions, indicating that the development of newborns was not affected by the change in ambient temperature. To the contrary, the color of interscapular fat was white in the group subjected to the warm condition, suggesting a higher lipid content. This result is consistent with studies showing that the morphology of BAT is largely affected by ambient temperature. For example, in adult mice, warm conditions, such as a thermoneutral temperature, induce brown adipocytes to change and take on a white adipocyte-like appearance (whitening of BAT) [14, 15]. A similar brown-to-white conversion is also observed in large mammals that lose BAT with aging: brown adipocytes gradually turn into white-like unilocular adipocytes [16, 17]. This age-dependent BAT whitening process is reported to be suppressed in guinea pigs in a cold environment [13], possibly due to the retention of UCP1 activity. Thus, it is plausible that the warm condition in our study suppressed UCP1 activity, resulting in the higher lipid content in the tissue.
We observed that the postnatal-day-dependent increase in Ucp1 expression and the proliferation of brown adipocyte progenitors were markedly suppressed in the warm condition. This suppression of BAT formation may be due to the reduction of sympathetic nerve activity in a warm environment [18], as it has been reported that norepinephrine content in BAT decreased in rats raised at 28 °C for 3 weeks after birth compared with that in rats raised at 23 °C [19]. It has also been reported that the cold-stimulation-induced proliferation of stromal cells in BAT is suppressed by the inhibitors of ß-adrenergic receptors in mice [20]. Thus, in developing hamsters, the proliferation of brown adipocyte progenitors may be stimulated by the sympathetic nerve–norepinephrine–ß-adrenergic receptor pathway in an ambient temperature-dependent manner. In addition, growth factors may also be involved because it has been reported that the cold-induced activation of the sympathetic nervous system increases the expression of several different growth factors, such as insulin-like growth factor 1, fibroblast growth factor 2, nerve growth factor, and Vegfa in BAT [21,22,23,24,25]. Some of these growth factors are reported to induce the proliferation of stromal-vascular cells in vivo [26, 27]. In our study, we found that Vegfa expression in the BAT was much lower in the warm condition. Consistent with the well-known role of vascular endothelial growth factor alpha (VEGFα) in vascularization, the proliferation of endothelial cells was suppressed and the blood vessel content was low in the warm condition. The reduction in vascularization observed in warm conditions plausibly suppresses the thermogenic ability of BAT in hamsters, as also previously reported in mice [4].
In this study, the warm condition largely and significantly suppressed the proliferation of brown adipocyte progenitors. However, the interscapular fat of 25-day-old hamsters consisted of differentiated brown adipocytes irrespective of ambient temperature, albeit the lipid content differed between the groups. We previously reported that hamster brown adipocytes arise through two different pathways: (1) the proliferation and differentiation of brown adipocyte progenitors and (2) the conversion of unilocular adipocytes to multilocular brown adipocytes [9]. Thus, it is possible that the latter pathway compensated for the suppression of the former pathway in the warm condition. It should also be noted that while the warm condition reduced the expression of both Ucp1 and Vegfa, it did not affect Cox4 expression, despite the expressions of these genes being reported in other animal species to be controlled by the sympathetic nervous system. Unlike mice and rats, hamsters are reported to increase BAT thermogenesis in response to cold exposure without increasing UCP1 expression or mitochondrial content [28]. Hamsters are hibernators and need to decrease their body temperature during hibernation, leading to the suggestion that hamsters differ from mice and rats in terms of sympathetic nerve innervation of BAT [29]. In addition, it was very recently reported that hamsters exhibit cold tolerance due to the expression of cold-insensitive transient receptor potential cation channel subfamily M member 8 (TRPM8), a cold-sensing channel, in the somatosensory neurons [30]. Further studies are needed to reveal how the ambient temperature and sympathetic nervous system is involved in BAT formation in hamsters.
Taking the findings of this study together, we suggest that the proliferation of brown adipocyte progenitors was suppressed in the warm condition, resulting in the attenuation of postnatal BAT formation in Syrian hamsters. The warm condition also decreased vascularization in the tissue due to the decrease in Vegfa expression and the proliferation of endothelial cells. Based on our results, it is possible that environmental temperature is an important physiological stimulus that promotes postnatal BAT formation in Syrian hamsters.
References
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
Cinti S (2005) The adipose organ. Prostaglandins Leukot Essent Fat Acids 73:9–15
Kajimura S, Saito M (2014) A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 76:225–249
Xue Y, Petrovic N, Cao R (2009) Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab 9:99–109
Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387:90–94
Jimenez M, Léger B, Canola K, Lehr L, Arboit P, Seydoux J, Russell A, Giacobino J, Muzzin P, Preitner F (2002) β1/β 2/β 3-Adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett 530:37–40
Houstĕk J, Janíková D, Bednár J, Kopecký J, Sebestián J, Soukup T (1990) Postnatal appearance of uncoupling protein and formation of thermogenic mitochondria in hamster brown adipose tissue. Biochim Biophys Acta 1015:441–449
Smalley RL, Smalley KN (1967) Brown and White fats; development in the hamster. Science 157:1449–1551
Okamatsu-Ogura Y, Nio-Kobayashi J, Nagaya K, Tsubota A, Kimura K (2018) Brown adipocytes postnatally arise through both differentiation from progenitors and conversion from white adipocytes in Syrian hamster. J Appl Physiol 124(1):99–108
Hissa R (1968) Postnatal development of thermoregulation in the norwegian lemming and the golden hamster. Ann Zool Fennici 5:345–383
Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP (2007) Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 48:41–51
Mouroux I, Bertin R, Portet R (1990) Thermogenic capacity of the brown adipose tissue of developing rats; effects of rearing temperature. J Dev Physiol 14:337–342
Holloway BR, Davidson RG, Freeman S, Wheeler H, Stribling D (1984) Post-natal development of interscapular (brown) adipose tissue in the guina pig- effect of environmental temperature. Obesity 8:295–303
Peng XR, Gennemark P, O’Mahony G, Bartesaghi S (2015) Unlock the thermogenic potential of adipose tissue: pharmacological modulation and implications for treatment of diabetes and obesity. Front Endocrinol (Lausanne) 6:174
Xiao C, Goldgof M, Gavrilova O, Reitman ML (2015) Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 °C. Obesity 23:1450–1459
Sakurai Y, Okamatsu-Ogura Y, Nakao R, Ohnuma A, Saito M, Kobayashi M, Kimura K (2015) Brown adipose tissue expressed uncoupling protein 1 in newborn harbor seals (Phoca vitulina). Mar Mamm Sci 31(2):818–827
Sellayah D, Sikder D (2014) Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology 155:485–501
Teramura Y, Terao A, Okada Y, Tomida J, Okamatsu-Ogura Y, Kimura K (2014) Organ-specific changes in norepinephrine turnover against various stress conditions in thermoneutral mice. Jpn J Vet Res 62:117–127
Beauvallet M, Portet R, Blancher G, Solier M (1978) Post-natal development of brown adipose tissue in the rat bred at 23 or 28 °C. Arch Int Physiol Biochim 86:145–152
Fukano K, Okamatsu-Ogura Y, Tsubota A, Nio-Kobayashi J, Kimura K (2016) Cold exposure induces proliferation of mature brown adipocyte in a β3-adrenergic receptor-mediated pathway. PLoS One 11:1–13
Asano A, Morimatsu M, Nikami H, Yoshida T, Saito M (1997) Adrenergic activation of vascular endothelial growth factor mRNA expression in rat brown adipose tissue: implication in cold-induced angiogenesis. Biochem J 328:179–183
Asano A, Kimura K, Saito M (1999) Cold-induced mRNA expression of angiogenic factors in rat brown adipose tissue. J Vet Med Sci 61:403–409
Duchamp C, Burton KA, Geloen A, Dauncey MJ (1997) Transient upregulation of IGF-I gene expression in brown adipose tissue of cold-exposed rats. Am J Physiol 272:E453–E460
Garcia B, Obregon MJ (1997) Norepinephrine potentiates the mitogenic effect of growth factors in quiescent peadipocytes:relationship with uncoupling protein messenger ribonucleic acid expression. Endocrinology 138:4227–4233
Geloen A, Collet AJ, Bukowiecki LJ (1992) Role of sympathetic innervation in brown adipocyte proliferation. Am J Physiol 263:1176–1181
Bagchi M, Kim LA, Boucher J, Walshe TE, Kahn CR, D’Amore PA (2013) Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J 27:3257–3271
Sung HK, Doh K, Son J, Park J, Bae Y, Choi S, Nelson S, Mary L, Cowling R, Nagy K, Michael I, Koh G, Adamson S, Pawson T, Nagy A (2013) Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17:61–72
Himms-Hagen J, Gwilliam C (1980) Abnormal brown adipose tissue in hamsters with muscular dystrophy. Am J Physiol 239:18–22
Himms-Hagen J (1984) Nonshivering thermogenesis. Brain Res Bull 12:151–160
Matos-Cruz V, Schneider ER, Mastrotto M, Merriman DK, Bagriantsev SN, Gracheva EO (2017) Molecular prerequisites for diminished cold sensitivity in ground squirrels and hamsters. Cell Rep 21:3329–3337
Funding
This study was supported by JSPS KAKENHI Grant Numbers 17K0811807 and 15H04545, Grants-in-Aid for the Naito Foundation, and for the Akiyama Life Science Foundation.
Author information
Authors and Affiliations
Contributions
Author contributions
KN, YOO, JNK, SN, and AT conducted the experiments; YOO and KK designed the experiments; KN and YOO wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were performed in accordance with the guidelines of Hokkaido University Manual for Implementing Animal Experimentation, in the animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The experimental procedures and care of animals were approved by the Animal Care and Use Committee of Hokkaido University (Hokkaido, Japan). This article does not contain any studies with human participants performed by any of the authors.
About this article
Cite this article
Nagaya, K., Okamatsu-Ogura, Y., Nio-Kobayashi, J. et al. Effect of ambient temperature on the proliferation of brown adipocyte progenitors and endothelial cells during postnatal BAT development in Syrian hamsters. J Physiol Sci 69, 23–30 (2019). https://doi.org/10.1007/s12576-018-0606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-018-0606-8