Abstract
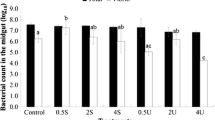
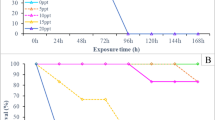
Cellular and humoral effectors are imperative for the innate defense mechanisms of invertebrates, including the spotted babylon snail Babylonia areolata, a marine gastropod belonging to the family Buccinidae. In this study, we have investigated the influence of body size [5–8 g (small), 10–12 g (medium) and 15–17 g (large)], and culture waters of varied quality [natural seawater, low salinity, low alkalinity, high total ammonia nitrogen (TAN) and artificial seawater] on some hemato-immunological parameters [total hemocytic counts (THC), hemolymphatic glucose, total protein concentration, phenoloxidase (PO), agglutinating and lysozyme activity] of the snail. Growth performance measures and survival of test snails were also evaluated after rearing them in culture water of varied quality. Body size did not influence most parameters assessed, except that the lysozyme and PO activities of medium and large-size snails were higher than those of the small-size snails. Sub-optimal culture water quality affected hemato-immunological factors, growth and/or survival of the spotted babylon snail. Seawater with low salinity, low alkalinity and a high TAN level caused decreased THC, hemolymphatic glucose, and PO activity; increased lysozyme activity; and clearly retarded growth of the snail. The snails held in artificial seawater could not survive after 4 weeks, suggesting the lack of certain essential factors necessary for their survival.



Similar content being viewed by others
References
Adema CM, Van der Knaap WPW, Siminia T (1991) Molluscan haemocyte-mediated cytotoxicity: the role of reactive oxygen intermediates. Rev Aquat Sci 5:201–223
Altena COVR, Gittenberger E (1981) The genus Babylonia (Prosobranchia, Buccinidae). Zool Verh 188:1–57
Atkinson M (1998) Elemental composition of commercial sea salts. J Aquaricult Aquat Sci 8:39
Bashevkin SM, Pechenik JA (2015) The interactive influence of temperature and salinity on larval and juvenile growth in the gastropod Crepidula fornicata L. J Exp Mar Biol Ecol 470:78–91
Boyd C, Thunjai T, Boonyaratpalin M (2002) Dissolved salts in waters for inland, low-salinity shrimp culture. GAA 5:40–45
Cawthorne DF, Beard T, Davenport J, Wickins JF (1983) Responses of juvenile Penaeus monodon Fabricius to natural and artificial sea waters of low salinity. Aquaculture 32:165–174
Chaitanawisuti N, Kritsanapuntu S, Santhaweesuk W (2009) Growth, production and economic considerations for commercial production of marketable sizes of spotted babylon Babylonia areolata, using a pilot abandoned marine shrimp hatchery and recirculating culture system. IJRA 10:43–62
Chaitanawisuti N, Kritsanapun S, Santhaweesuk W (2011a) Growth, food efficiency, and biochemical composition of juvenile spotted babylon Babylonia areolata Link fed on conventional trash fish and a formulated moist diet. Aquac Int 19:865–872
Chaitanawisuti N, Kritsanapuntu A, Santaweesuk W (2011b) Comparisons between two production–scale methods for the intensive culture of juveniles spotted babylon Babylonia areolata, to marketable sizes. Int J Fish Aquac 3:79–88
Chang YP, Liu CH, Wu CC, Chiang CM, Lian JL, Hsieh SL (2012) Dietary administration of zingerone to enhance growth, non-specific immune response, and resistance to Vibrio alginolyticus in Pacific white shrimp Litopenaeus vannamei juveniles. Fish Shellfish Immunol 32:284–290
Cheng W, Hsiao IS, Chen JC (2004a) Effect of ammonia on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Fish Shellfish Immunol 17:193–202
Cheng WT, Hsiao IS, Chen JC (2004b) Effect of nitrite on immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Dis Aquat Org 60:157–164
Cheng WT, Juang FM, Chen JC (2004c) The immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus at different salinity levels. Fish Shellfish Immunol 16:295–306
Clark MS, Husmann G, Thorne MAS, Burns G, Truebano M, Peck LS, Abele D, Philipp EER (2013) Hypoxia impacts large adults first: consequences in a warming world. Glob Change Biol 19:2251–2263
Davis DA, Saoud IP, McGraw WJ, Rouse DB (2002) Considerations for Litopenaeus vannamei reared in inland low salinity waters. In: Cruz-Suarez LE et al. (eds) Avances en Nutrición Acuícola. VI. Memorias del VI Simposium Internacional de Nutricion Acuicola. 3 al 6 de Septiembre del 2002, Cancun, Quintana Roo, Mexico, pp 73–90
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21:363–373
Di GL, Zhang ZX, Ke CH, Guo JR, Xue M, Ni JB, Wang DX (2011) Morphological characterization of the haemocytes of the ivory snail Babylonia areolata Neogastropoda Buccinidae. J Mar Biol Assoc UK 91:1489–1497
Di G, Zhang Z, Ke C (2013) Phagocytosis and respiratory burst activity of haemocytes from the ivory snail Babylonia areolata. Fish Shellfish Immunol 35:366–374
Galloway TS, Depledge MH (2001) Immunotoxicity in invertebrates: measurement and ecotoxicological relevance. Ecotoxicology 10:5–23
Guo Y, He H (2014) Identification and characterization of a goose-type lysozyme from sewage snail Physa acuta. Fish Shellfish Immunol 39:321–325
Hooper C, Day R, Slocombe R, Handlinger J, Benkendorff K (2007) Stress and immune responses in abalone: limitations in current knowledge and investigative methods based on other models. Fish Shellfish Immunol 22:363–379
Hossain MS, Hussain MZ, Chowdhury SR (2006) An analysis of economic and environmental issues associated with sea salt production in Bangladesh and Thailand coast. Int J Ecol Environ Sci 32:159–172
Huchette S, Koh CS, Day R (2003) Growth of juvenile blacklip abalone Haliotis rubra in aquaculture tanks: effects of density and ammonia. Aquaculture 219:457–470
Husmann G, Abele D, Rosenstiel P, Clark MS, Kraemer L, Philipp EER (2014) Age-dependent expression of stress and antimicrobial genes in the hemocytes and siphon tissue of the Antarctic bivalve Laternula elliptica exposed to injury and starvation. Cell Stress Chaperones 19:15–32
Hyvarinen A, Nikkila E (1962) Specific determination of blood glucose with O-toludine. Clin Chem Acta 7:140–143
Kovac N, Glavas N, Dolenec M, Smuc NR, Slejkovec Z (2013) Chemical composition of natural sea salt from the Secovlje Salina (Gulf of Trieste, northern Adriatic). Acta Chim Slov 60:706–714
Kritsanapuntu S, Chaitanawisuti N, Santhaweesuk W, Natsukari SY (2006) Effects of water exchange regimes on growth, survival and shell normality of the hatchery reared juvenile spotted babylon Babylonia areolata Link 1807 in a recirculating seawater system. Aquac Int 14:587–594
Kritsanapuntu S, Chaitanawisuti N, Santhaweesuk W, Natsukari Y (2008) Growth performances for monoculture and polyculture of hatchery-reared juvenile spotted babylon Babylonia areolata Link 1807, in large-scale earthen ponds. Aquac Res 39:1556–1561
Kritsanapuntu S, Chaitanawisuti N, Natsukari Y (2009) Growth and water quality for growing-out of juvenile spotted babylon Babylonia areolata, at different water-exchange regimes in a large-scale operation of earthen ponds. Aquac Int 17:77–84
Kritsanapuntu S, Chaitanawisuti N, Santaweesuk W (2013) Effects of dietary partial replacement of tuna oil by corn oil in formulated diets for growth performance and proximate composition of juvenile spotted babylon Babylonia areolata under hatchery conditions. J Aquac Res Dev 4:197
Loker ES (2010) Gastropod immunobiology. Adv Exp Med Biol 708:17–43
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lucas A (1996) Physical concepts of bioenergetics. In: Lucas A (ed) Bioenergetics of aquatic animals. Taylor and Francis, France
Panichasuk P (1996) Areola babylon Babylonia areolata Link 1807. Thai Fish Gaz 49:107–117
Ruangsri J, Lokesh J, Fernandes JMO, Kiron V (2014) Transcriptional regulation of antimicrobial peptides in mucosal tissues of Atlantic cod Gadus morhua L. in response to different stimuli. Aquac Res 45:1893–1905
Santarem M, Figueras A (1995) Basic studies on defense mechanisms of mussels. In: Stolen JS et al (eds) Techniques in fish immunology 4. Immunology and pathology of aquatic invertebrates, SOS, Fair Haven
Soderhall K, Rogener W, Soderhall I, Newton RP, Ratcliffe NA (1988) The properties and purification of a Blaberus craniifer plasma protein which enhances the activation of haemocyte prophenoloxidase by a β 1, 3-glucan. Insect Biochem 18:323–330
Sung HH, Chang HJ, Her CH, Chang JC, Song YL (1998) Phenoloxidase activity of hemocytes derived from Penaeus monodon and Macrobrachium rosenbergii. J Invertebr Pathol 71:26–33
Supamattaya K, Ruangsri J, Kiriratnikom S, Suanyuk N (2002) Normal immuno-physiological parameters in black tiger shrimp Penaeus monodon and the effects of environmental stress and diseases. Fish Sci 68:983–984
Vedamanikam VJ, Hayimad T (2013) Effect of mixtures of metals on the spotted babylon snail Babylonia areolata under different temperature conditions. Toxicol Environ Chem 95:1388–1394
Xue M, Ke CH, Wang DX, Wei YJ, Xu YB (2010) The combined effects of temperature and salinity on growth and survival of hatchery-reared juvenile spotted babylon Babylonia areolata Link 1807. J World Aquac Soc 41:116–122
Acknowledgements
This research was financially supported by Prince of Songkla University, Surat Thani Campus, Thailand. Nirut Sukasem and his staff at Rajabhut Phuket University are gratefully acknowledged for their help in conducting the experiments with sub-optimal water quality conditions. We thank Associate Prof. Dr. Seppo Karrila for checking the English language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruangsri, J., Thawonsuwan, J., Wanlem, S. et al. Effect of body size and sub-optimal water quality on some hemato-immunological parameters of spotted babylon snail Babylonia areolata. Fish Sci 84, 513–522 (2018). https://doi.org/10.1007/s12562-018-1191-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1191-8