Abstract
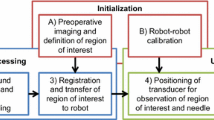
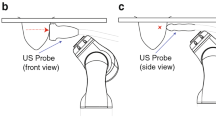
We present a compact and lightweight two degrees-of-freedom (DOF) needle end-effector to be applied to a teleoperated needle interventional robotic system. The suggested needle end-effector is computerized tomography (CT)-compatible and easy to sterilize. Because the proposed needle end-effector can be attached to a robotic manipulator, the needle can translate into and rotate in the human body. In addition, a 1-axis load cell is attached to the needle end-effector to measure the longitudinal force applied to the needle. With this force measurement, force-feedback ability has been implemented in the entire master-slave robotic system. Basic performance tests of repeatability and maximum insertion force were performed. Several force-feedback experiments to determine free space response and contact response, as well as a discrimination test, were conducted. Through this verification process, the suggested needle end-effector was found to have potential for application in real environments that require robotic CT-guided needle interventions.
Similar content being viewed by others
References
F. Heinrich, F. Joeres, K. Lawonn, and C. Hansen, “Comparison of projective augmented reality concepts to support medical needle insertion,” IEEE Trans. Vis. Comput. Graph., vol. 25, no. 6, pp. 2157–2167, 2019.
A. K. Han, J. H. Bae, K. C. Gregoriou, C. J. Ploch, R. E. Goldman, G. H. Glover, B. L. Daniel, and M. R. Cutkosky, “MR-compatible haptic display of membrane puncture in robot-assisted needle procedures,” IEEE Trans. Haptics, vol. 11, no. 3, pp. 443–454, 2018.
M. Khadem, C. Rossa, N. Usmani, R. S. Sloboda, and M. Tavakoli, “Robotics-assisted needle steering around anatomical obstacles using notched steerable needles,” IEEE J. Biomed. Health Inform., vol. 22, no. 6, pp. 1917–1928, 2018.
B. Xu, C. Zhou, and S. Y. Ko, “Closed-loop planar fuzzy control system for a curvature-controllable steerable beveltip needle,” Int. J. Control. Autom., vol. 16, no. 5, pp. 2421–2431, 2018.
G. S. Fischer, I. Iordachita, C. Csoma, and G. Fichtinger, “MRI-compatible pneumatic robot for transperineal prostate needle placement,” IEEE/ASME Trans. on Mechatron., vol. 13, no. 3, pp. 295–305, 2008.
A. V. DAmico, R. Cormack, C. M. Tempany, S. Kumar, G. Topulos, H. M. Kooy, and C. N. Coleman, “Real-time magnetic resonance image-guided interstitial brachytherapy in the treatment of select patients with clinically localized prostate cancer,” Int. Journal of Radiat. Oncol. Biol. Phys., vol. 42, pp. 507–515, 1998.
S. Zangos, K. Eichler, K. Engelmann, and T. J. Vogl, “MR-guided transgluteal biopsies with an open low-field system in patients with clinically suspected prostate cancer: Technique and preliminary results,” Eur. Radiol., vol. 15, no. 1, pp. 174–182, 2005.
S. E. Song, N. B. Cho, G. Ficsher, N. Hata, C. M. Tempany, G. Fichtinger, and I. Iordachita, “Development of a pneumatic robot for MRI-guided transperineal prostate biopsy and brachytherapy: new approaches,” In Proc. IEEE Int. Conf. Robotics and Automation, pp. 2580–2585, 2010.
R. Kokes, K. Lister, R. Gullapalli, B. Zhang, A. MacMillan, H. Richard, and J. Desai, “Towards a teleoperated needle driver robot with haptic feedback for RFA of breast tumors under continuous MRI,” Med. Image Anal., vol. 13, no. 3, pp. 445–455, 2009.
K. Y. Kim, H. S. Woo, J. H. Cho, and Y. K. Lee, “Development of a two DOF needle driver for CT-guided needle insertion-type interventions,” Proc. of IEEE Int. Sym. Robot and Human Interactive Communication, pp. 470–475, 2017.
Y. S. Kwoh, J. Hou, E. Jonckheere, and S. Hayati, “A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery,” IEEE Trans. Biomed. Eng., vol. 35, no. 2, pp. 153–160, 1988.
D. Stoianovici, L. Whitcomb, J. Anderson, R. Taylor, and L. Kavoussi, “A modular surgical robotic system for image guided percutaneous procedures,” Medical Image Computing and Computer-Assisted Intervention, Cambridge, USA, 1998.
K. Masamune, G. Fichtinger, A. Patriciu, R. Susil, R. Taylor, L. Kavoussi, J. Anderson, I. Sakuma, T. Dohi, and D. Stoianovici, “System for robotically assisted percutaneous procedures with computer tomography guidance,” Comput. Aided Surg., vol. 6, pp. 370–383, 2001.
B. Maurin, B. Bayle, O. Piccin, J. Gangloff, M. de Mathelin, C. Doignon, P. Zanne, and A. Gangi, “A patient-mounted robotic platform for CT-scan guided procedures,” IEEE Trans. Biomed. Eng., vol. 55, no. 10, pp. 2417–2425, 2008.
Y. Moon, J. B. Seo, and J. Choi, “Development of new end-effector for proof-of-concept of fully robotic multichannel biopsy,” IEEE/ASME Trans. Mechatron., vol. 20, no. 6, pp. 2996–3008, 2015.
H. Cha, J. Chung, W. K. Kim, and B. Yi, “Master-slave robotic system for 3 dimensional needle steering,” Proc. of IEEE/RSJ Int. Conf. Intelligent Robots and Systems, pp. 857–862, 2010.
Q. C. Nguyen, Y. Kim, and H. Kwon, “Optimization of layout and path planning of surgical robotic system,” Int. J. Control. Autom., vol. 15, no. 1, pp. 375–384, 2017.
D. D. Lorenzo, Y. Koseki, E. D. Momi, K. Chinzei, and A. M. Okamura, “Coaxial needle insertion assistant with enhanced force feedback,” IEEE Trans. Biomed. Eng., vol. 60, no. 2, pp. 379–389, 2012.
O. Piccin, L. Barbe, B. Bayle, M. de Mathelin, A. Gangi, “A force feedback teleoperated needle insertion device for percutaneous procedures,” Int. J. Robot. Res., vol. 28, no. 9, pp. 1154–1168, 2009.
H. Song, K. Kim, and J. Lee, “Development of optical fiber bragg grating force-reflection sensor system of medical application for safe minimally invasive robotic surgery,” Rev. Sci. Instrum., vol. 82, no. 7, pp. 074301–8, 2011.
A. J. Madhani, G. Niemeyer, and J. K. Salisbury Jr., “The Black Falcon: A teleoperated surgical instrument for minimally invasive surgery,” in Proc. IEEE/RSJ Int. Conf. Intelligent Robots and Systems, Victoria, Canada, pp. 936–944, 1998.
K. Kim, J. Lee, “Design and evaluation of a slave manipulator with roll-pitch-roll wrist and automatic tool loading mechanism in telerobotic surgery,” Int. J. Med. Robot., vol. 8, no. 4, pp. 421–435, 2012.
H. A. Mansy, J. R. Grahe, and R. H. Sandler, “Elastic properties of synthetic materials for soft tissue modeling,” Phys. Med. Biol., vol. 53, no. 8, pp. 2115–2130, 2008.
R. M. Murray, Z. Li, and S. S. Sastry, A Mathematical Introduction to Robotic Manipulation, CRC Press, Berkeley, 1994.
M. Dede and S. Tosunoglu, “Fault-tolerant teleoperation systems design,” Ind. Robot., vol. 33, no. 5, pp. 365–372, 2006.
Y. L. Park, S. Elayaperumal, B. Daniel, S. C. Ryu, M. Shin, J. Savall, R. J. Black, B. Moslehi, and M. R. Cutkosky, “Real-time estimation of 3-D needle shape and deflection for MRI-guided interventions,” IEEE/ASME Trans. Mechatron., vol. 15, no. 6, pp. 906–914, 2010.
L. Barbe, B. Bernard, M. de Michel, and G. Afshin, “In vivo model estimation and haptic characterization of needle insertions,” Int. J. Robot. Res., vol. 26, pp. 1283–1301, 2007.
P. Kim, S. Kim, S. H. Choi, J. S. Oh, and S. B. Choi, “Force modeling for incision surgery into tissue with haptic application,” SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring. International Society for Optics and Photonics; SPIE: Bellingham, WA, USA, 2015.
S. R. Lee, C. H. Uhm, M. S. Seong, J. S. Oh, S. B. Choi, “Repulsive force control of minimally invasive surgery robot associated with three degrees of freedom electrorheological fluid-based haptic master,” Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci., vol. 228, pp. 1606–1621, 2013.
C. Yang, Y. Xie, S. Liu, and D. Sun, “Force modeling, identification, and feedback control of robot-assisted needle insertion: a survey of the literature,” Sensors, vol. 18, no. 561, pp. 1–38, 2018.
S. Jiang, P. Li, Y. Yu, J. Liu, and Z. Yang, “Experimental study of needle-tissue interaction forces: effect of needle geometries, insertion methods and tissue characteristics,” J. Biomech., vol. 47, pp. 3344–3353, 2014.
J. Suh and K. Kim, “Design of a discrete bending joint using multiple unit PREF joints for isotropic 2-DOF motion,” Int. J. Control. Autom., vol. 15, no. 1, pp. 64–72, 2017.
C. Ju and H. I. Son, “Evaluation of haptic feedback in the performance of a teleoperated unmanned ground vehicle in an obstacle avoidance scenario,” Int. J. Control. Autom., vol. 17, no. 1, pp. 168–180, 2019.
A. J. Madhani, Design of Teleoperated Surgical Instruments for Minimally Invasive Surgery, Ph.D. Thesis, MIT, 1998.
K. Ogata, Modern Control Engineering, 4th ed., Prentice Hall, 2003.
K. B. Reed, A. M. Okamura, and N. J. Cowan, “Modeling and control of needles with torsional friction,” IEEE Trans. Biomed. Eng., vol. 56, no. 12, pp. 2905–2916, 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Recommended by Guest Editors Doo Yong Lee (KAIST) and Jaesoon Choi (Asan Medical Center). This work was supported by the Industrial Strategic Technology Development Program, Grant Number 10077502, Development of an Intelligent Cardiovascular Intervention Assist Robot System with 3-Dimensional Cardiac Mapping System and Blood Vessel Visualization, and by Grant Number NK217E, Development of Highly Efficient and Safe Industrial Manipulator for Human–Robot Collaboration. All applicable international, national, or institutional guidelines for the care and use of animals were followed.
Kiyoung Kim received his Ph.D. in Mechanical Engineering from Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea, in 2012. He is a Senior Researcher with the Department of Medical Assistant Robotics, Korea Institute of Machinery & Materials (KIMM). He was a Postdoctoral Research Associate at the Hamlyn Centre, Imperial College London, UK. His research interests are primarily in the areas of surgical manipulators, continuum robotics, and human-robot physical interactions.
Hyunsoo Woo received his Ph.D. in Mechanical Engineering from Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea, in 2009. He is currently a Principal Investigator and Head of the Department of Medical Assistant Robotics, Korea Institute of Machinery & Materials (KIMM). He was a Postdoctoral Researcher at KAIST. His research interests include surgical/rehabilitation robotics, robotic prostheses, and industrial robotic manipulators.
Jang Ho Cho received his M.S. and a Ph.D. in Mechanical Engineering from Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea, in 2004 and 2010, respectively. From 2011 to 2013, he was a Linneaus Postdoctoral Researcher with the Lund Center for Control of Complex Engineering Systems, Department of Automatic Control, Lund University, Sweden. He is currently a Senior Researcher in the Department of Medical Assistant Robotics, Korea Institute of Machinery & Materials (KIMM). His research interests include time delay control, teleoperation, haptic interactions, and parallel robots for medical applications.
Jungwook Suh received his B.S., M.S., and Ph.D. degrees in Mechanical Engineering from Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea, in 2007, 2009, and 2013, respectively. From 2014 to 2019, he worked as a senior researcher at the Electronics and Telecommunications Research Institute (ETRI). He is currently an Assistant Professor at the Department of Robot and Smart System Engineering, Kyungpook National University (KNU), Daegu, Korea. His research interests include robotics and mechanism design for medical devices and robots.
Rights and permissions
About this article
Cite this article
Kim, K., Woo, H., Cho, J.H. et al. Design, Modeling, and Evaluation of a Compact and Lightweight Needle End-effector with Simple Force-feedback Implementation for Robotic CT-guided Needle Interventions. Int. J. Control Autom. Syst. 18, 85–101 (2020). https://doi.org/10.1007/s12555-019-0235-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12555-019-0235-x