Abstract
Background
Pediatric post coronavirus disease 2019 (COVID-19) condition (PPCC) is a heterogeneous syndrome, which can significantly affect the daily lives of children. This study aimed to identify clinically meaningful phenotypes in children with PPCC, to better characterize and treat this condition.
Methods
Participants were children with physician-diagnosed PPCC, referred to the academic hospital Amsterdam UMC in the Netherlands between November 2021 and March 2023. Demographic factors and information on post-COVID symptoms, comorbidities, and impact on daily life were collected. Clinical clusters were identified using an unsupervised and unbiased approach for mixed data types.
Results
Analysis of 111 patients (aged 3–18 years) revealed three distinct clusters within PPCC. Cluster 1 (n = 62, median age = 15 years) predominantly consisted of girls (74.2%). These patients suffered relatively more from exercise intolerance, dyspnea, and smell disorders. Cluster 2 (n = 33, median age = 13 years) contained patients with an even gender distribution (51.5% girls). They suffered from relatively more sleep problems, memory loss, gastrointestinal symptoms, and arthralgia. Cluster 3 (n = 16, median age = 11 years) had a higher proportion of boys (75.0%), suffered relatively more from fever, had significantly fewer symptoms (median age of 5 years compared to 8 and 10 years for clusters 1 and 2 respectively), and experienced a lower impact on daily life.
Conclusions
This study identified three distinct clinical PPCC phenotypes, with variations in sex, age, symptom patterns, and impact on daily life. These findings highlight the need for further research to understand the potentially diverse underlying mechanisms contributing to post-COVID symptoms in children.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In March 2020, the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) outbreak a global pandemic [1], affecting millions of people worldwide. COVID-19 is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and while the initial focus was on pulmonary symptoms, it is now well established that COVID-19 can have diverse disease presentations and involve multiple organ systems [2]. Although children are less likely to experience severe disease than adults, they can still be affected by COVID-19 and develop long-term symptoms [3,4,5]. The persistence of symptoms for longer than 12 weeks after COVID-19 is known as post-COVID-19 condition [6]. This condition in children will henceforth be referred to as pediatric post-COVID-19 condition (PPCC).
The reported occurrence of PPCC varies widely among studies, with a large systematic review [7] from May 2023 reporting a pooled prevalence of 23%, with prevalence rates ranging from 3.7% to 66% [7,8,9]. This wide range could be caused by differences in study populations (e.g., hospitalized versus. non-hospitalized children), different follow-up approaches, or lack of control groups [10]. PPCC is a heterogeneous illness, characterized by a broad range of symptoms and the involvement of multiple organ systems. The most common complaints are fatigue, mood changes, headache, cognitive difficulties (“brain fog”), dyspnea, and loss of smell [4, 10]. Other symptoms related to PPCC include tachycardia, chest pain, cough, abdominal pain, nausea and lack of appetite, (recurrent) fever, post-exertional malaise, sleep dysfunction, dizziness, skin rashes, and joint and muscle pains [11]. Risk factors for long-term symptoms after COVID-19 in children that have previously been described are older age (> 10 years), female gender, comorbidities (e.g., allergies, neurologic or genetic diseases), and hospitalization during the acute phase [12, 13].
PPCC can have a profound effect on daily life [12], necessitating a growing societal demand for diagnostic and treatment strategies. This has led to the emergence of PPCC clinics all over the world and an international knowledge exchange platform, consisting of physicians, allied-health professionals, and patient representatives [14]. However, the treatment strategies employed vary greatly among these clinics and are mostly based on experience. This is partly associated with a lack of understanding of the pathophysiological mechanisms underlying PPCC. Recent research hypothesizes that post-COVID-19 condition in adults may be caused by immune dysregulation (e.g., due to viral persistence), microbiota disruption, autoimmunity, clotting and endothelial abnormality, or dysfunctional neurologic signaling [15]. However, it is unclear if similar mechanisms are responsible for post-COVID-19 condition in children.
From clinical practice, we observed a diversity of symptoms associated with PPCC [14], yet certain characteristics seem to cluster together. Buonsenso et al. [16] described symptom patterns, grouping them (supervised clustering) based on organ systems. Unsupervised clustering of symptoms in adults with post-COVID-19 condition on separate occasions has shown that such a group can be clustered into phenotypes with different organ system involvement and severity [17, 18]. However, this has not yet been performed in children with PPCC. Unsupervised clustering of PPCC symptoms and characteristics could provide inherent patterns that may represent distinct phenotypes.
The aim of this study was to identify clinically meaningful phenotypes of PPCC. Understanding the clinical features of PPCC may help us to better characterize this condition, thereby expanding our knowledge of potential underlying pathophysiological mechanisms.
Methods
Study design
This study used data collected in the post-COVID syndrome (POCOS) study, a prospective, observational cohort study conducted at the Amsterdam University Medical Center (Amsterdam UMC), a tertiary care hospital based in Amsterdam, the Netherlands. The POCOS study investigates PPCC in the pediatric population, aiming to describe clinical characteristics, investigate underlying mechanisms, and identify biomarkers for PPCC. Data from patients was collected from medical files and during patient visits. The medical ethics committee of the Amsterdam UMC, location AMC, approved the study (METc 2021_126). All participants and/or caregivers provided oral and written informed consent.
Participants and procedures
From November 2021 to March 2023, Dutch children (aged 0–18 years) with PPCC referred to the post-COVID multidisciplinary outpatient clinic of the Amsterdam UMC were consecutively invited to participate in the POCOS study. The inclusion criteria were: (1) PCC, as diagnosed by a physician according to the WHO definition [6], with a history of at least one positive real-time reverse transcription-polymerase chain reaction (RT-PCR) test on nasopharyngeal, oropharyngeal, sputum, or fecal sample for SARS-CoV-2, or proof of infection with SARS-CoV-2 by positive serology test (immunoglobulin G (IgG)/immunoglobulin M (IgM)), or medical history suitable with acute SARS-CoV-2 infection, if the acute episode occurred between February to June 2020 (due to restricted testing possibilities for children in the Netherlands), and (2) complaints lasting > 12 weeks after acute COVID-19.
Data collection
Demographic and clinical characteristics
Demographic factors, including information on age, sex, level of education, and clinical characteristics such as medical history, comorbidities, symptoms in the acute COVID-19 phase, post-COVID symptoms, and body mass index (BMI) plus Z-scores [19] were collected from all participants, either by outpatient visits with a physician or retrieved from medical files. The suspected SARS-CoV-2 variant was determined by the dominant virus type in the Netherlands at the time of infection based on numbers from the National Institute for Public Health and the Environment (RIVM) [20]. Limitations in daily life were scored for three domains: first, school limitations, with impact scores of 0 (no impact), 1 (mild impact; 1–2 days of absence per month), 2 (moderate impact; 1–2 days of absence per week), and 3 (severe impact; 3–5 days of absence per week). Second, social limitations, with impact scores of 0 (no impact), 1 (mild impact; 1–2 days per month not capable), 2 (moderate impact; 1–2 days per week not capable), and 3 (severe impact; no social contact possible). Third, physical limitations, with impact scores of 0 (no impact), 1 (mild impact; 80% of physical functioning), 2 (moderate impact; 50% of physical functioning), and 3 (severe impact; no physical functioning).
Patient-reported outcomes measurement information system (PROMIS) pediatric fatigue questionnaire
Fatigue is the most common symptom of post-COVID-19 condition [5]. PROMIS® Pediatric Fatigue Scale is a questionnaire that uses the Computerized Adaptive Test (CAT) format, in which subsequent questions are chosen based on the answers given earlier. PROMIS Fatigue scores are reported on a T-score metric ranging from 0 to 100, with higher values representing more fatigue [21]. The PROMIS Fatigue has been validated among Dutch children (between 8 and 18 years of age), who showed an average T-score of 39.8 ± 12.4 [22]. For this reason, only children aged ≥ 8 years old received questionnaires. The minimal clinically important difference (MCID) of the PROMIS Fatigue is 2–6 points [23].
Statistical analyses
Data storage
All collected data was stored in an online case report form (CRF) by Castor Electronic Data Capture (EDC) [24], a platform intended for capturing medical research data in clinical trials. The study was closely monitored and the quality of the data was controlled.
Imputation
An overview of the variables used for clustering is found in Supplementary Table 1. Variables were only eligible for selection if at most 15% were missing. Missing data was imputed using the Multiple Imputation by Chained Equations (MICE) algorithm as implemented by the mice R package (version 3.15.0) [25]. Imputation for numerical variables was performed using predictive mean matching, binary categorical variables were imputed with logistic regression, ordered categorical variables were imputed with a proportional odds model, and unordered variables were imputed through polytomous logistic regression. One hundred different imputed datasets were created to account for the uncertainty of missing data.
Clustering algorithm
The individual datasets were converted to pairwise Gower distance between the patients. Hierarchical clustering based on the Ward.D2 construction method was used to create dendrograms. Visual inspection of the dendrograms and the Dunn index [26] determined the optimal number of clusters that maximize the distances between clusters while minimizing the distances within clusters. To obtain a consensus clustering, pairwise distances from the cluster assignments in individual datasets were calculated for each pair of patients, which was subsequently clustered through hierarchical clustering.
Cluster interpretation
Patient demographics and clinical characteristics were compared between the clusters using statistical tests based on the type of data. Categorical variables were compared using Fisher’s exact test, and numerical variables were compared with the Kruskal–Wallis test. Post-hoc testing of the statistical significance of comparisons was performed with pairwise Fisher’s exact tests for categorical variables and pairwise Wilcoxon rank sum tests with correction for multiple testing for numerical variables. Symptom presence or absence between the two largest clusters was compared with an odds ratio as calculated by the epitools package (version 0.5–10.1) [27]. All analyses were performed in R (version 4.1.2) with RStudio (version 2021.09.1 + 372) [28].
Results
Cohort demographics
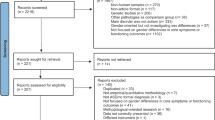
In total, 111 participants were included in this study (Fig. 1). The demographic and clinical characteristics of these patients can be found in Table 1. Missing data constituted 1.9% of all data used for clustering. Participants were between 3 and 18 years old, with a median age of 14.0 years [interquartile range (IQR): 11.0–16.0]. The cohort consisted of slightly more girls than boys (60.4% and 39.6% respectively). The most common complaints were fatigue (100.0%), exercise intolerance (80.2%), and oversensitivity/overstimulation (76.6%). Over two-thirds (68.5%) of the participants reported comorbidities. The BMI was normal for children of these ages, with a median z-score of − 0.1 (IQR − 0.7–0.9). All participants had mild acute COVID-19; none were admitted to the hospital during the acute phase.
Differences in patient demographics and comorbidities
Clusters were formed through hierarchical clustering. Based on visual inspection of the dendrograms and the Dunn indices from the clustering of individual datasets, and from the consensus clustering (Supplementary Fig. 1), three clusters were chosen to best represent the data. Cluster separation based on a t-SNE plot can be seen in Fig. 2. In total, 62 patients were placed in cluster 1, 33 patients in cluster 2, and 16 patients in cluster 3. A comparison between the clusters can be found in Table 1. The clusters showed differences in age and sex distribution (P < 0.001). Cluster 1 predominantly consisted of girls (74.2%), cluster 2 was more evenly distributed (48.5% boys), while cluster 3 predominantly consisted of boys (75.0%); however, this was not statistically different from cluster 2 or from 50% (P = 0.077). Cluster 1 also contained relatively older patients, with a median age of 15.0 years (IQR: 13.0–16.0), compared to a median age of 13.0 years (IQR: 9.0–16.0) in cluster 2 and 11.0 years (IQR: 8.8–14.0) in cluster 3. In terms of comorbidities, there were no differences between the clusters for asthma, however, cluster 3 contained significantly fewer patients with allergies (18.8% compared to 50.0% and 60.6% in cluster 1 and 2, respectively) and cluster 2 contained significantly fewer patients with psychiatric disorders (6.1% compared to 25.8% and 31.2% in cluster 1 and 3, respectively).
Differences in patterns of symptoms
The clusters showed differences in symptom patterns (Fig. 3). The most common symptoms are summarized in Fig. 4. Cluster 3 showed the lowest median number of symptoms per patient, with a median of five symptoms, compared to eight and ten for cluster 1 and 2 respectively (P < 0.001). The only symptom found more commonly in cluster 3 compared to the other clusters was fever (P = 0.042).
Comparing the patients’ symptoms between clusters 1 and 2 revealed two distinct symptom patterns. Symptoms that are significantly more common in cluster 1 include exercise intolerance [P = 0.022, odds ratio (OR) = 5.06, 1.26–26.41], smell disorder (P = 0.032, OR = 3.33, 1.10–12.74), and dyspnea (P = 0.040, OR: 2.49, 1.04–6.06). Patients in cluster 2 suffered significantly more from sleep problems (P < 0.001, OR = 19.48, 6.45–75.94), abdominal pain (P < 0.001, OR = 11.52, 4.17–35.60), nausea (P < 0.001, OR = 10.64, 4.06–30.45), loss of appetite (P = 0.001, OR = 4.32, 1.77–11.01), arthralgia (P = 0.024, OR: 3.31, 1.17–9.79), and memory loss (P = 0.032, OR = 2.80, 1.09–8.00). Myalgia was also more common in this cluster but failed to reach statistical significance (P = 0.081, OR: 2.35).
Differences in terms of impact on daily life and fatigue severity
The impact of daily-life scores followed the distribution of the number of symptoms per cluster. More symptoms correlated with a higher impact on the daily life of participants. In terms of the impact on school and exercise, patients from cluster 3 indicated that they experience significantly less impact compared to the other clusters. However, this difference was not statistically significant for social interactions, and neither were any differences found between clusters 1 and 2. For the fatigue severity as determined through the PROMIS questionnaire, cluster 3 (score: 57.0 ± 8.2) showed signs of suffering less from fatigue than the other clusters, while no significant difference (P = 0.077) was found between clusters 1 (score: 63.7 ± 9.4) and 2 (score: 63.7 ± 12.0). Given that all patients indicated that they suffer from fatigue, all scores were significantly higher than the average score in Dutch children (39.8 ± 12.4) [22].
Little distinction between clusters
Other demographic data or data related to acute COVID-19 severity showed less distinction between the clusters. No significant differences were found in the time between the infection and study visit, vaccination rate, and whether they were vaccinated before or after SARS-CoV-2 infection. We found no differences in the suspected SARS-CoV-2 variant or the number of times children were infected. While there is a significant difference in the level of education of patients, this is mainly related to age, with the younger cluster 3 showing a higher rate of elementary scholars. This difference was nullified when patients going to elementary school were excluded from the analysis (P = 0.30). Patients from cluster 2 showed a slightly higher rate of developing gastroenteritis during acute COVID-19, although this was not statistically significant (P = 0.056), while patients from cluster 3 showed a significantly higher rate of an acute COVID-19 presentation classified as “other”.
Discussion
In this study, we clustered Dutch patients suffering from PPCC into clinically distinct phenotypes. We discovered three phenotypes that show significant differences in terms of sex, age, symptom patterns, and impact on daily life. Fatigue was the most commonly reported symptom for the entire cohort, underlined by the average PROMIS Fatigue score being significantly higher than the average score for healthy Dutch children [22].
We found three distinct phenotypes of PPCC in our pediatric population, which is in line with a study conducted in adult patients with post-COVID-19 condition by Kenny et al.[17] This study reported one cluster with more cardiorespiratory symptoms, one cluster with more musculoskeletal pain, and one cluster with a significantly lower number of symptoms and burden of disease. This may suggest that disease presentation and underlying pathophysiology of post-COVID-19 condition could be similar in children and adults. However, other studies performing cluster analyses in adult populations with post-COVID-19 condition show contrasting results, identifying post-COVID-19 condition phenotypes with different characteristics [18, 29,30,31]. This might be due to differences in data collection methods (self-reported versus validated questionnaires) or variations in the duration since infection (long-term symptoms after > 4 weeks versus after > 12 months). However, it could also highlight the heterogeneity of the disease, emphasizing the necessity of independent validation of cluster analyses.
Two previously described risk factors for post-COVID-19 condition in adults and children are female sex [13, 18, 31] and older age [12]. In our cohort, we found similar characteristics to be potentially associated with a higher burden of disease, as evidenced by cluster 3 mainly consisting of younger boys who experienced the least symptoms and reported the lowest impact on daily life. Another previously described predictor for PPCC is severe acute COVID-19 with or without hospitalization during the acute phase [12, 32], but because our cohort only included children with mild acute clinical presentation of COVID-19, we could not investigate this risk factor in our study.
Post-COVID symptoms had a mild to severe self-reported impact on three domains of daily life (school, social interactions, and physical functioning) for almost all our participants. This is in line with findings in a cohort of Hungarian children with PPCC [33]. However, social restrictions and lockdowns during the pandemic can also affect mental and physical health [34] and must be taken into consideration when assessing the impact of PPCC symptoms on daily life.
PPCC is a heterogeneous disease, for which the identification of three distinct clinical phenotypes may advance our understanding of the underlying pathophysiological mechanisms of the disease. One hypothesis could be that autonomic dysfunction or viral reservoirs found in the brain might play a central role in cluster 1, characterized by symptoms such as dyspnea, exercise intolerance, and neurocognitive problems [15, 35, 36]. Another hypothesis for cardiorespiratory symptoms such as dyspnea and exercise intolerance could be persistent lung inflammation, as was recently described in an adult population with PCC [37]. Cluster 2 reported high numbers of gastrointestinal complaints such as abdominal pain, nausea, and loss of appetite, which could be a result of gut microbial dysbiosis, viral persistence, or altered neuro-immune interactions in the gut [15, 38]. In cluster 3, (recurrent) fever was a common symptom, which could be a result of autoimmunity, immune dysregulation due to chronic inflammation, or dysautonomia [15, 39]. Nevertheless, this cluster had a small sample size of only 16 patients, making it challenging to draw generalizable conclusions. All described theories need further in-depth investigations, preferably through (randomized) trials.
Our study has limitations. First, we only invited children who were referred to our tertiary care clinic to participate in our study. This means we only included patients with severe PPCC symptoms, which has created a selection bias, lowering the generalizability of our results. In addition, because this was a real-time study, there was no standard timeframe after infection that children were seen for the study visit, which explains the large variability in days since infection. Furthermore, because the POCOS study included children who were referred for standard care reasons, not all data was collected for every patient, which explains why there is missing data for some participants (e.g., the PROMIS Fatigue questionnaires). Second, the selection of the number of clusters was based on visual inspection of the clustering dendrogram instead of statistical methods. For the individual imputed datasets, the Dunn index [26] suggested a wide range for the number of clusters but selected three clusters most often. Finally, our cohort consisted of 111 patients, which is a relatively small sample size to perform cluster analyses on, hence why we were unable to perform validation analyses within this study cohort.
On the other hand, we were able to perform an unbiased hierarchical clustering analysis on a population with physician-diagnosed PPCC with mild acute SARS-CoV-2 infection, where alternative diagnoses were excluded. Another strength of our study lies in the scope of information we collected, providing a complete view of our participants, while biologic samples collected in the POCOS study allow for molecular characterization of the clusters in future analyses.
Classification of PPCC phenotypes can aid in comprehending its progression, identifying its causes, and ultimately developing management strategies tailored to specific phenotypes. In addition, the identification of phenotypes can help determine appropriate, personalized rehabilitation treatment strategies for children with PPCC.
Validation of these cluster analyses in a larger population is recommended to increase generalizability. Machine learning-based clustering has previously been applied to identify potential PPCC patients based on their clinical records [40], and has also been employed in the identification of PCC phenotypes in adults [30]. However, this method has not yet been performed for PPCC cluster analyses, making it a potentially promising tool. Further biomedical research, e.g., with a multi-omics approach, is needed to determine the possible underlying pathophysiology associated with these phenotypes.
In conclusion, PPCC is a heterogeneous and poorly characterized illness that can significantly affect the lives of children. This study found three distinct clinical phenotypes of PPCC that show differences in terms of gender, age, symptom patterns, and impact on daily life. These phenotypes may reflect different underlying pathophysiological mechanisms for post-COVID symptoms, which could help categorize patients for more successful monitoring and treatment strategies, as well as funnel future research into potential cluster targets.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/europe/emergencies/situations/covid-19. Accessed October 23, 2023.
To KK, Sridhar S, Chiu KH, Hung DL, Li X, Hung IF, et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg Microbes Infect. 2021;10:507–3510.
Pierce CA, Herold KC, Herold BC, Chou J, Randolph A, Kane B, et al. COVID-19 and children. Science. 2022;377:1144–9.
Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, Perelman C, Sepulveda R, Rebolledo PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950.
Brackel CLH, Lap CR, Buddingh EP, van Houten MA, van der Sande L, Langereis EJ, et al. Pediatric long-COVID: An overlooked phenomenon? Pediatr Pulmonol. 2021;56:2495–502.
World Health Organization (WHO). A clinical case definition of post COVID-19 condition by a delphi consensus. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed October 23, 2023.
Zheng YB, Zeng N, Yuan K, Tian SS, Yang YB, Gao N, et al. Prevalence and risk factor for long COVID in children and adolescents: a meta-analysis and systematic review. J Infect Public Health. 2023;16:660–7210.
Pellegrino R, Chiappini E, Licari A, Galli L, Marseglia GL. Prevalence and clinical presentation of long COVID in children: a systematic review. Eur J Pediatr. 2022;181:3995–4009.
Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–11.
Behnood S, Newlands F, O’Mahoney L, Haghighat Ghahfarokhi M, Muhid MZ, Dudley J, et al. Persistent symptoms are associated with long term effects of COVID-19 among children and young people: results from a systematic review and meta-analysis of controlled studies. PLoS ONE. 2023;18:e0293600.
Wacks M, Wortley E, Gregorowski A, Segal TY, Whittaker E. Fifteen-minute consultation: managing post-COVID-19 syndrome (long COVID) in children and young people. Arch Dis Child Educ Pract Ed. 2024;109:29–34.
Morello R, Mariani F, Mastrantoni L, De Rose C, Zampino G, Munblit D, et al. Risk factors for post-COVID-19 condition (Long Covid) in children: a prospective cohort study. EClinicalMedicine. 2023;59:101961.
Jiang L, Li X, Nie J, Tang K, Bhutta Z. A systematic review of persistent clinical features after SARS-CoV-2 in the pediatric population. Pediatrics. 2023;152:2.
Brackel CLH, Noij LCE, Vijverberg SJH, Legghe CL, Maitland-van der Zee AH, van Goudoever JB, et al. International Care programs for Pediatric Post-COVID Condition (Long COVID) and the way forward. Pediatr Res. 2024. https://doi.org/10.1038/s41390-023-03015-0.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46.
Buonsenso D, Espuny Pujol F, Munblit D, Pata D, McFarland S, Simpson FK. Clinical characteristics, activity levels and mental health problems in children with long coronavirus disease: a survey of 510 children. Future Microbiol. 2022;17:577–88.
Kenny G, McCann K, O’Brien C, Savinelli S, Tinago W, Yousif O, et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect Dis. 2022;9:ofac060.
Gentilotti E, Gorska A, Tami A, Gusinow R, Mirandola M, Rodriguez Bano J, et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. 2023;62:102–7.
Myatt M, Guevarra E. zscorer: Child Anthropometry z-Score Calculator. R package version 0.3.1. 2019. https://CRAN.R-project.org/package=zscorer. Accessed October 23, 2023.
National Institute for Public Health and the Environment (RIVM). Tabel kiemsurveillance COVID-19. 2023. https://www.rivm.nl/documenten/tabel-kiemsurveillance-covid-19. Accessed October 23, 2023.
Food and Drugs Administration (FDA). PROMIS Fatigue Scoring Manual. 2017. https://www.fda.gov/media/137977/download. Accessed October 23, 2023.
Peersmann SHM, Luijten MAJ, Haverman L, Terwee CB, Grootenhuis MA, van Litsenburg RRL. Psychometric properties and CAT performance of the PROMIS pediatric sleep disturbance, sleep-related impairment, and fatigue item banks in Dutch children and adolescents. Psychol Assess. 2022;34:860–9.
Terwee CB, Peipert JD, Chapman R, Lai JS, Terluin B, Cella D, et al. Minimal important change (MIC): a conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual Life Res. 2021;30:2729–54.
Castor EDC. https://data.castoredc.com. Accessed June 24, 2023.
van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Dunn JC. A fuzzy relative of the ISODATA process and itsuse in detecting compact well-separated clusters. J Cybernet. 1973;3:32–57.
Aragon TJ. epitools: epidemiology tools. R package version 0.5–10.1. 2020. https://cran.r-project.org/web/packages/epitools/index.html. Accessed October 23, 2023.
R Core Team. R: a language and environment for statistical computing: R Foundation for Statistical Computing; 2021. https://www.R-project.org/. Accessed November 3, 2023.
Fischer A, Badier N, Zhang L, Elbeji A, Wilmes P, Oustric P, et al. Long COVID classification: findings from a clustering analysis in the Predi-COVID Cohort Study. Int J Environ Res Public Health. 2022;19:16018.
Reese JT, Blau H, Casiraghi E, Bergquist T, Loomba JJ, Callahan TJ, et al. Generalisable long COVID subtypes: findings from the NIH N3C and RECOVER programmes. EBioMedicine. 2023;87:104413.
Goldhaber NH, Kohn JN, Ogan WS, Sitapati A, Longhurst CA, Wang A, et al. Deep dive into the long haul: analysis of symptom clusters and risk factors for post-acute sequelae of COVID-19 to inform clinical care. Int J Environ Res Public Health. 2022;19:16841.
Izquierdo-Pujol J, Moron-Lopez S, Dalmau J, Gonzalez-Aumatell A, Carreras-Abad C, Mendez M, et al. Post COVID-19 condition in children and adolescents: an emerging problem. Front Pediatr. 2022;10:894204.
Garai R, Krivacsy P, Herczeg V, Kovacs F, Tel B, Kelemen J, et al. Clinical assessment of children with long COVID syndrome. Pediatr Res. 2023;93:1616–25.
Glinianowicz M, Ciura D, Burnatowska E, Olszanecka-Glinianowicz M. Psychological effects of the COVID-19 pandemic - what do we know about them? Eur Rev Med Pharmacol Sci. 2023;27:6445–58.
Larsen NW, Stiles LE, Miglis MG. Preparing for the long-haul: autonomic complications of COVID-19. Auton Neurosci. 2021;235:102841.
Proal AD, VanElzakker MB, Aleman S, Bach K, Boribong BP, Buggert M, et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat Immunol. 2023;24:1616–27.
Paris D, Palomba L, Albertini MC, Tramice A, Motta L, Giammattei E, et al. The biomarkers’ landscape of post-COVID-19 patients can suggest selective clinical interventions. Sci Rep. 2023;13:22496.
Meringer H, Mehandru S. Gastrointestinal post-acute COVID-19 syndrome. Nat Rev Gastroenterol Hepatol. 2022;19:345–6.
Gu J, Liu Q, Zhang J, Xu S. COVID-19 and trained immunity: the inflammatory burden of long covid. Front Immunol. 2023;14:1294959.
Lorman V, Razzaghi H, Song X, Morse K, Utidjian L, Allen AJ, et al. A machine learning-based phenotype for long COVID in children: an EHR-based study from the RECOVER program. PLoS ONE. 2023;18:e0289774.
Acknowledgements
We thank all the patients who participated in this study and their families.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
NLCE: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing—original draft, and writing—review and editing. BJM: conceptualization, data curation, formal analysis, methodoloy, software, validation, visualization, writing—original draft, and writing—review and editing. LCR: conceptualization, data curation, investigation, methodology, project administration, resources, and writing—review and editing. HMA: conceptualization, funding acquisition, methodology, project administration, resources, supervision, and writing—review and editing. BG: conceptualization, funding acquisition, methodology, project administration, resources, supervision, and writing—review and editing. MZAH: supervision, and writing—review and editing. AAMI: supervision, and writing—review and editing. GJB: writing—review and editing. AMW: investigation, and writing—review and editing. BCLH: conceptualization. OKJ: investigation. HS: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, and writing—review and editing. T-LSWJ.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
NLCE, BJM, LCR, HMA, BG, AAMI, AMW, BCLH, OKJ, HS and TLSWJ have nothing to declare. M-ZAH is the PI of a public private consortium [P4O2 (Precision Medicine for More Oxygen)] sponsored by Health Holland involving many private partners that contribute in cash and/or in kind (AbbVie. Boehringer Ingelheim, Breathomix, Clear, Fluidda, Ortec Logiqcare, Olive, Philips, Quantib-U, Smartfish, Clear, SODAQ, Thirona, Roche, TopMD, Novartis, RespiQ). She received unrestricted research grants from GSK and Boehringer Ingelheim and received the Vertex innovation award grant, and had honoraria paid to institution by GSK, Boehringer Ingelheim and Astra Zeneca. MZAH is also the chair of DSMB of a study on BPD in neonates and the president of FIGON (Federation Innovative drug research in the Netherlands). GJB has received a grant from Danone Research and has a patent planned for amino acid composition of infant formulas. He is a member of the national health council (unpaid) and the director of the national Human Milk Bank (unpaid).
Ethical approval
The medical ethics committee of the Amsterdam UMC, location AMC, approved the study (METc 2021_126). Informed consent to participate in the study has been obtained from all participants (or their parent or legal guardian in the case of children under 16).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noij, L.C.E., Blankestijn, J.M., Lap, C.R. et al. Clinical-based phenotypes in children with pediatric post-COVID-19 condition. World J Pediatr (2024). https://doi.org/10.1007/s12519-024-00805-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12519-024-00805-2