Abstract
Introduction
The MYH7 c.5135G > A p.(Arg1712Gln) variant has been identified in several patients worldwide and is classified as pathogenic in the ClinVar database. We aimed to delineate its associated phenotype and evaluate a potential founder effect.
Methods
We retrospectively collected clinical and genetic data of 22 probands and 74 family members from an international cohort.
Results
In total, 53 individuals carried the MYH7 p.(Arg1712Gln) variant, of whom 38 (72%) were diagnosed with hypertrophic cardiomyopathy (HCM). Mean age at HCM diagnosis was 48.8 years (standard deviation: 18.1; range: 8–74). The clinical presentation ranged from asymptomatic HCM to arrhythmias (atrial fibrillation and malignant ventricular arrhythmias). Aborted sudden cardiac death (SCD) leading to the diagnosis of HCM occurred in one proband at the age of 68 years, and a family history of SCD was reported by 39% (5/13) probands. Neither heart failure deaths nor heart transplants were reported. Women had a generally later-onset disease, with 14% of female carriers diagnosed with HCM at age 50 years compared with 54% of male carriers. In both sexes, the disease was fully penetrant by age 75 years. Haplotypes were reconstructed for 35 patients and showed a founder effect in a subset of patients.
Conclusion
MYH7 p.(Arg1712Gln) is a pathogenic founder variant with a consistent HCM phenotype that may present with delayed penetrance. This suggested that clinical follow-up should be pursued after the seventh decade in healthy carriers and that longer intervals between screening may be justified in healthy women < 30 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
A large series of patients with the MYH7 p.(Arg1712Gln) variant showed: (1) a consistent hypertrophic cardiomyopathy (HCM) phenotype, (2) women developing HCM at a later age, and (3) development of HCM after the sixth decade.
-
Describing a series of individuals with a single pathogenic variant is important to establish variant-specific screening protocols to prevent unnecessary cardiac evaluations.
-
For healthy MYH7 p.(Arg1712Gln) carriers, longer screening intervals could be appropriate for women younger than 30 years and follow-up should be continued after the age of 60 years.
-
Our data also showed that MYH7 p.(Arg1712Gln) is a founder variant in a subset of French and Dutch patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most prevalent inherited cardiomyopathy, affecting 1 in 500 individuals in the general population. HCM is defined by hypertrophy of the left ventricular walls (≥ 15 mm in sporadic cases and ≥ 13 mm in the presence of family history) that is not explained by loading conditions. It is characterised by an increased risk of arrhythmias and sudden cardiac death (SCD).
A large number of genes have been associated with HCM, of which only 11 have definitive or moderate evidence to be associated with HCM [1]. The number of carriers of a given variant is usually small, and it is not easy to establish precise genotype-phenotype correlations for each variant, often making the individualised management of carriers challenging. Using clinical and molecular data from an international cohort, we aimed to delineate the clinical phenotype associated with the MYH7 c.5135G > A p.(Arg1712Gln) variant, gain more evidence for its pathogenicity, and evaluate a potential founder effect.
Methods
Subjects
We retrospectively collected clinical and molecular data from 22 apparently unrelated probands who carried the p.(Arg1712Gln) variant in the MYH7 gene (NM_000257.4) and 74 family members. Informed consent for publication was obtained from all patients or their legal representatives in accordance with the Declaration of Helsinki and national legal regulations.
Molecular data
Analysis of cardiomyopathy-related genes was performed in 15 probands (68%) using targeted next-generation sequencing (NGS) analysis. The minimal NGS gene set included the following genes: MYH7, MYBPC3, MYL2, TNNT2 and TNNI3. Single-gene analysis using Sanger sequencing and/or denaturing high-performance liquid chromatography of the following sets of genes was reported for probands evaluated before 2013: MYH7 and MYBPC3 (3 probands (14%)), MYH7, MYBPC3, MYL2, MYL3, TNNI3, TPM1 and TNNT2 (3 probands (14%)), and MYH7, MYBPC3, TNNI3, TPM1, TNNT2 and GLA (1 proband (4%)). Cascade genetic testing was performed on family members when requested. Interpretation of variants was performed according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) guidelines [2] and the adapted ACMG/AMP criteria for MYH7 [3].
Clinical data
Clinical data were retrieved retrospectively from medical records. Clinical, electrocardiographic and echocardiographic/magnetic resonance imaging (MRI) data were collected for all patients. HCM was diagnosed according to the European Society of Cardiology HCM guidelines [4]. Particular attention was paid to the following clinical variables: age at HCM diagnosis, hypertension, atrial fibrillation, stroke, syncope, permanent pacemaker or implanted cardioverter-defibrillator (ICD) implantation and therapy, septal reduction therapy, cardiac transplantation, heart failure (HF), and family history of HCM or SCD. Age at HCM diagnosis was used as surrogate for penetrance [5].
Haplotype analysis
Assessment of ancestry via haplotype analysis was conducted in 25 patients from the Netherlands and France. Haplotype data were available for 10 additional Dutch carriers for whom no clinical data were available, and they were not included in the cohort. We studied 15 dinucleotide repeat markers spanning an 8.7-Mb region on both sides of MYH7 (see Table S1 in Electronic Supplementary Material). Protocols are available upon request.
To establish the phase of alleles, haplotypes were determined with co-segregation analysis of family members. When no family members were available, haplotypes were derived when the available allele matched a previously determined disease-associated haplotype as performed in a previous study [6].
Results
Molecular data
The MYH7 p.(Arg1712Gln) variant was detected in all probands (n = 22). No other (likely) pathogenic variant was identified. Among the 73 family members who underwent cascade screening, 30 carried the MYH7 variant. One additional family member (patient #8.17) was an obligate carrier by pedigree history.
Clinical data
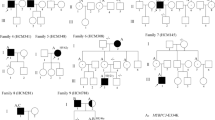
Phenotype data were available for 22 probands and 74 family members (Tab. 1 and Table S2).
Probands
Of the 22 probands, 10 (45%) were females. Mean (± standard deviation) age at HCM diagnosis was 46.7 years (range: 8–74) in all probands, 38.6 years (range: 8–66) in males and 59.3 years (range: 34–74) in females. Mean age at last examination was 61.4 ± 19.3 years. Follow-up data were available for 77% (17/22) of the probands, with a median follow-up duration of 9 years after HCM diagnosis (range: 1–42).
At the time of diagnosis, mean maximal wall thickness was 17.5 ± 2.5 mm (range: 13–21), left ventricular outflow tract obstruction (LVOTO) was detected in 57% (4/7) of probands, and 2 additional probands had LVOTO but the age of onset was unknown. The most frequently reported initial manifestations were chest pain or tightness (5/11; 45%) and dyspnoea (3/11; 27%).
Patients #12 (male) and #14 (female) were asymptomatic at diagnosis. HCM was accidentally diagnosed in patient #12 at age 76 years during routine cardiologic evaluation following a fracture. Patient #14 was diagnosed with HCM at 40 years of age during cardiac screening for SCD family history. In 1 female proband (patient #7), the presenting symptom was SCD during moderate physical activity at age 68 years.
Atrial fibrillation was observed in 7/14 probands (41%). Two probands underwent septal reduction therapy, 2 developed a TIA, and 1 developed chronic HF. No HF deaths nor heart transplants were recorded. ICD implantation was reported for 3 probands at age 59 years in a male and at ages 68 and 72 years in 2 females.
A positive family history of HCM was reported for 79% (11/14) of probands. Five of 13 probands (38%) had a family history of SCD, occurring in 7 family members (2 females (29%)) at a mean age of 35 years (range: 18–48). Age at SCD and sex were unknown for 2 additional family members.
Family members
Of the 74 family members, 30 (14 females (47%)) tested positive for the familial p.(Arg1712Gln) variant and 1 was an obligate carrier. Of these 31 carriers, 16 (52%) were diagnosed with HCM, 6 (38%) of whom were females. Median age at HCM diagnosis in family members was 58 years (range: 20–72), and mean maximal wall thickness at the time of diagnosis was 16.5 ± 2.2 mm (range: 13–20). Follow-up data were available for 6 family members, with a median follow-up duration of 8.5 years after HCM diagnosis (range: 2–47). In genotype-positive relatives, the HCM phenotype varied widely, ranging from asymptomatic HCM to more severe disease requiring myectomy. None of the family members needed heart transplantation or died because of HF. Mean age at last examination of carriers without HCM was 42 ± 15.5 years (range: 21–64).
Penetrance
Overall penetrance of HCM in our cohort was 72%, and this was sex- and age-dependent (Figs. 1 and 2). The earliest age at diagnosis was 8 years, and no women were diagnosed with HCM before age 34 years. Forty-four percent (14/32) of the individuals, half of whom were women, were diagnosed with HCM at age ≥ 60 years. While female probands were significantly older at HCM diagnosis than male probands (mean age: 59.3 ± 12.4 years vs 38.6 ± 18.2 years; p = 0.0063), there was no statistically significant difference in age at diagnosis between sexes among family members (mean age: 58 ± 17.7 years in women vs 49 ± 18.8 years in men; p = 0.1812).
Haplotype analysis
DNA for haplotype analysis was available for 25 patients from 18 unrelated families (4 families from France and 14 from the Netherlands) and 10 additional Dutch carriers for whom we had no clinical data and who were not included in our clinical cohort (see Table S3 in Electronic Supplementary Material). We found 3 haplotype groups, which were all absent in relatives without the variant (data not shown). The largest group showed shared haplotypes of ≥ 4 markers, covering at least a 2-Mb region surrounding MYH7 in 10 Dutch and all 4 French families, as well as in 8 additional Dutch carriers. Several recombination events occurred in this group, which are indicated by different colours in Table S3 in the Electronic Supplementary Material. In the second group, 3 Dutch patients shared haplotypes of ≥ 6 markers spanning a 2.2-Mb region.
Discussion
We have described the phenotype associated with the MYH7 p.(Arg1712Gln) variant in 22 probands and 31 family members, the largest cohort of individuals with this specific variant and the second largest cohort with a single MYH7 variant to date. We have shown it is a founder variant with classical elements of inherited cardiac disease, i.e. clinical variability (yet a consistent HCM phenotype) and age-dependent penetrance.
Two major observations were made: the penetrance was overall delayed for both sexes compared with the MYH7 subset from the HCM SHaRe registry (n = 492) [5], and no woman developed HCM before the fourth decade. In our cohort and in the MYH7 subset of the HCM SHaRe registry, age at diagnosis was 58.8 ± 12.2 years and 34.8 ± 19.2 years in women (p < 0.0001), respectively, and 43.6 ± 18.7 years and 33.3 ± 16.8 years in men (p = 0.0028), respectively [5].
In the SHaRe registry, women and men developed HCM caused by different MYH7 variants at similar ages (n = 492; 34.8 ± 19.2 years in women vs 33.3 ± 16.8 years in men; p = 0.39) [5], whereas we observed a statistically significant difference in age at diagnosis between sexes in our cohort as a whole (n = 53; 58.8 ± 12.2 years in women vs 43.6 ± 18.7 years in men; p = 0.0011) and in the probands (p = 0.0063). Nevertheless, there was no statistically significant difference in age at HCM diagnosis between sexes among family members (p = 0.1812), which could be explained by unknown genetic or clinical modifiers, a delay in reporting symptoms and/or a delay in cardiology referral in women who are not known to be at high risk of developing HCM (e.g. in the absence of a family history of HCM or SCD), as observed for other cardiac conditions [7,8,9].
In our cohort, only 1 patient was diagnosed with HCM before the age of 10 years. He underwent septal reduction by myectomy at 8 years of age and presented with chronic HF at the age of 43 years. Genetic screening of 23 HCM genes using NGS did not identify any additional disease-causing variant in this patient, but a second genetic HCM-causing variant that contributed to this early onset of the disease cannot be fully excluded.
Severity of HCM in our cohort varied largely, spanning from asymptomatic HCM to symptomatic and life-threatening arrythmias, with aborted SCD and HF reported in a female proband and history of SCD in 39% of the families. All relatives who did not carry the variant (n = 43) were not affected by HCM: 79% (n = 34) were evaluated using echocardiography and/or cardiac MRI, 12% (n = 5) were evaluated using electrocardiography only, and cardiac clinical screening was reported as normal in 9% (n = 4) without additional information.
The MYH7 p.(Arg1712Gln) variant affects a highly conserved residue within the myosin tail domain and is predicted to be probably damaging to the protein structure/function PolyPhen‑2, deleterious by MutationTaster and by SIFT. This variant is present in gnomAD (http://gnomad.broadinstitute.org, accessed on 14 April 2023), with an allele frequency of 0.00002125 (6/282354). The 6 mutated alleles reported in gnomAD are all detected in the European, non-Finnish population at the heterozygous state in individuals who are in the 45–70-year age range. This variant is classified as pathogenic in the ClinVar database according to the ‘Expert Panel designation’ (https://www.ncbi.nlm.nih.gov/clinvar, accession number VCV000036642.42, accessed on 14 April 2023), and our data provide additional supporting evidence for its pathogenicity. Although it has been reported to be associated with HCM in the literature (Tab. 2), as far as we know we are the first to prove its founder effect.
Implications
This study showed that studying a large series of individuals with a single specific variant underlying an inherited cardiac disease may lead to observations different from the general picture associated with a specific disease or its underlying gene or variant, as we have shown previously for other genes/variants [10]. Therefore, we propose a variant-specific approach for MYH7 p.(Arg1712Gln) carriers.
Current guidelines recommend precautionary regular cardiac evaluations in healthy carriers of HCM-causing variants as the disease may appear late in adulthood [11, 12]. Clinical screening of healthy carriers is usually performed every 1–3 years in childhood and every 2–5 years in adulthood, according to national and international guidelines [4, 13, 14]. Since no women in our cohort were diagnosed with HCM before age 34 years, longer intervals between follow-up examinations could be considered for women < 30 years with this specific variant.
It remains difficult to establish the age at which to discontinue cardiac follow-up in genotype-positive, phenotype-negative individuals. According to French national guidelines, clinical follow-up is usually stopped in healthy carriers > 60 years, when the risk of developing the disease is considered to be low [13], whereas a Dutch national guideline working group states that there are insufficient data to establish an age limit for long-term follow-up of healthy carriers of HCM-causing variants [14]. Since 6/13 probands (4 women) and 4/31 family members (1 woman) were diagnosed with HCM after the age of 60 years, we suggest that cardiac follow-up of healthy carriers of the MYH7 p.(Arg1712Gln) variant should be continued after the seventh decade.
Study limitations
The limitations of our study include its retrospective nature and the non-standardised assessment of patients since clinical information was obtained from patient files from multiple centres across 2 countries. Moreover, limited clinical data were available for a number of patients.
References
Ingles J, Goldstein J, Thaxton C, et al. Evaluating the clinical validity of hypertrophic cardiomyopathy genes. Circ Genom Precis Med. 2019;12:e2460.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Kelly MA, Caleshu C, Morales A, et al. Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: recommendations by ClinGen’s Inherited Cardiomyopathy Expert Panel. Genet Med. 2018;20:351–9.
Authors/Task Force members, Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79.
Lakdawala NK, Olivotto I, Day SM, et al. Associations between female sex, sarcomere variants, and clinical outcomes in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2021;14:e3062.
Van Lint FHM, et al. Arrhythmogenic right ventricular cardiomyopathy-associated desmosomal variants are rarely de novo. Circ Genom Precis Med. 2019;12:e2467.
Khera S, Kolte D, Gupta T, et al. Temporal trends and sex differences in revascularization and outcomes of ST-segment elevation myocardial infarction in younger adults in the United States. J Am Coll Cardiol. 2015;66:1961–2.
Bhave PD, Lu X, Girotra S, et al. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–12.
Joyce DL, et al. Disparities in access to left ventricular assist device therapy. J Surg Res. 2009;152:111–7.
Hoorntje ET, et al. Lamin A/C-related cardiac disease: late onset with a variable and mild phenotype in a large cohort of patients with the Lamin A/C p.(Arg331Gln) founder mutation. Circ Cardiovasc Genet. 2017;10:e1631.
Wilde AAM, Semsarian C, Márquez MF, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. Europace. 2022;24:1307–67.
Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2020;76:3022–55.
Protocole National de Diagnostic et de Soins (PNDS) Cardiomyopathie Hypertrophique. https://www.has-sante.fr, French. Accessed 14 April 2023.
Genetische diagnostiek en erfelijkheidsadvisering bij Hypertrofische Cardiomyopathie (HCM). https://richtlijnendatabase.nl, Dutch. Accessed 14 April 2023.
Members of The European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart (ERN GUARD-Heart; http://guardheart.ern-net.eu)
Luisa Marsili, Freyja H. M. van Lint, J. Peter van Tintelen, Arjan C. Houweling, Ronald H. Lekanne Deprez, Arthur A. M. Wilde, Dennis Dooijes, Jan G. Post, Irma van de Beek, Alexa M. C. Vermeer, Karin Y. van Spaendonck-Zwarts, Flavie Ader, Pascale Richard, Bertrand Isidor, Marie-Line Bichon, Sandra Mercier.
Funding
This work was supported by the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation (Hartstichting; CardioVasculair Onderzoek Nederland (CVON) projects: PREDICT2 2018-30, DOSIS 2014-40, DOUBLE-DOSE 2020B005).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
L. Marsili, F.H. M. van Lint, F. Russo, K.Y. van Spaendonck-Zwarts, F. Ader, M.-L. Bichon, L. Faivre, A.C. Houweling, B. Isidor, R.H. Lekanne Deprez, M.G. P. J. Cox, A.A. M. Wilde, B. Mazel, S. Mercier, D. Dooijes, G. Millat, K. Nguyen, J.G. Post, P. Richard, I. van de Beek, A.M. C. Vermeer, L. Boven, J.D. H. Jongbloed, J. P. van Tintelen and The European Reference Network for Rare and Low Prevalence Complex Diseases of the Heart declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marsili, L., van Lint, F.H.M., Russo, F. et al. MYH7 p.(Arg1712Gln) is pathogenic founder variant causing hypertrophic cardiomyopathy with overall relatively delayed onset. Neth Heart J 31, 300–307 (2023). https://doi.org/10.1007/s12471-023-01798-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-023-01798-9