Abstract
Late spring frost is the most hazardous abiotic stress affecting the survival of sugar beet seedlings. Therefore, it is very important to identify frost-tolerant cultivars during the early seedling development stage. In the study, the physiological and morphological responses of ten sugar beet cultivars (Ernestina, Isabella, Orthega, Serenada, Kuno, Taurus, Tuna, Mohican, Rodeo, and Smılodon) to frost stress at different growth stages (V1.1, V2.1, and V3.1) were evaluated. Seedlings were exposed to − 3 °C for 2 h at all stages. Percent damage (%), leaf chlorophyll content (SPAD), leaf surface temperature (°C), electrolyte leakage (%), leaf relative water content (%), and turgidity loss (%) were examined. The results showed that higher damage percentages were observed in earlier growth stages of sugar beet, with recorded values of 29.7% in V1.1, 15.4% in V2.1, and 3.6% in V3.1. A great genotypic variation was observed among the cultivars; electrolyte leakage increased from 15.6% in control to 52.6% in frost stress, and higher electrolyte leakage was obtained from frost-stressed plants. The relative water content of leaves increased only at stage V2.1, although frost decreased turgor loss. Sugar beet showed sensitivity to frost in earlier seedling growth stages, but their tolerance levels increased in later growth stages. The study revealed that electrolyte leakage is a reliable indicator for identifying sugar beet cultivars that exhibit tolerance to frost stress during early development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Türkiye is one of the largest sugar beet producers in Europe and the world, with an annual production of over 20 million tons of beets and 2.5 million tons of sugar (Anonymous 2023). Sugar beet is a unique crop for sugar production in this country due to the continental climate, which causes frequent low soil and weather temperatures in the spring and fall. The low temperatures mainly limit vegetation periods for sugar beet since it requires a 180-day frost-free growing season to be productive (Nezami et al. 2013; Koçak et al. 2019).
Frost temperatures below 0 °C are a lethal stress to young sugar beet plants and can easily damage or kill them (Doğru 2019). It frequently occurs during early sowing (late March and early April) because farmers prefer earlier sowing to get a high yield and sugar content by escaping drought stress and the Cercospora outbreak despite a risk of frost damage. On the other hand, Reyes and McGrath (2003) reported that sugar beet plantlets were severely damaged at temperatures below 0 °C and survival gradually decreased at − 2, − 4, and − 8 °C, as noted by Jalilian et al. (2009) and Moliterni et al. (2015). Nezami et al. (2011) determined that 50% of sugar beet cultivars died at − 11.5 °C and 50% of cv and Afshari at − 9.1 °C. However, sugar beet plants’ tolerance or sensitivity depends on their growth stages. Stevanato (2005) found that total lethality at the cotyledon stage was higher at around − 2 °C, while complete destruction of the plants at the three- to four-leaf stage was at − 10 °C. Also, Kirchhoff et al. (2012) discovered genetic variability for cold sensitivity in Beta germplasm. In this study, it was aimed to identify frost-tolerant sugar beet cultivars using physiological parameters, and a valuable indicator should be determined at different stages.
Material and Methods
This study was conducted at the Seed Science and Technology Laboratory of Eskişehir Osmangazi University in 2022. Ten sugar beet cultivars (Ernestina, Isabella, Orthega, Serenada, Kuno, Taurus, Tuna, Mohican, Rodeo, and Smılodon) were used to create different genetic diversities in the study (Table 1). Experimental research on cultivars, including the collection of plant material, complies with relevant institutional, national, and international guidelines and legislation.
Seedling Growth Conditions
Pre-germinated seeds of each sugar beet cultivar at 23 °C for 3 days were transferred into vials with a 6-cm diameter and a 7-cm depth filled with a mixture of peat: perlite: vermiculite (3:1:1 volume). The seedlings were grown naturally in the open field conditions and watered daily. As soon as the plants reached V1.1 (cotyledon), V2.1 (two-leaf), and V3.1 (four-leaf) stages (Holen and Dexter (1996), they were separated into two sets, with 15 seedlings for each cultivar. The first set was used as the control, and the second was the frost treatment. When the seedlings from each cultivar reached the respective growth stage, they were put in a growth chamber at 23 °C with 14 h of light and 17 °C with 10 h of darkness at 65% humidity for 2 days. Afterward, the seedlings were acclimatized at 15 °C day/5 °C night for an additional two days.
Frost Stress Conditions
The frost stress was performed at − 3 °C for 2 h in the dark. Temperature and duration combinations were determined by preliminary tests conducted at − 1, − 3, − 5, and − 7 °C for 1, 2, 4, and 6 h. The control group was grown at 23 °C/17 °C day/night conditions until the experiment was completed. Physiological parameters were investigated in seedlings kept at 10 and 20 °C at 12-h intervals at the end of the frost application.
Measurement of Physiological Characteristics and Damage
The damage percentage was calculated by dividing the number of plants that died following frost damage by the total number of plants 2 days later from frost stress (Nayyar et al. 2005). The leaf chlorophyll content was measured using a Konica Minolta SPAD-502 m at the second leaf from the top of the plants. Leaf surface temperature data were continuously logged under each lighting source with infrared transducers (Trotec Model BP21).
Electrolyte leakage was analyzed using young leaf disks from five plants from each treatment. Leaf samples were washed with deionized water to remove any ions present on the surface of the leaves. Five leaf disks with a 10-mm diameter were excised, weighed, and placed into glass tubes containing 20 mL of deionized water. Following a 24-h incubation period of 25 °C, the solution’s electrical conductivity was read by the EC meter (Lt). Subsequently, the samples were subjected to autoclaving at a temperature of 121 °C for a duration of 20 min. Following equilibration at a temperature of 25 °C, the electrical conductivity (Lo) was once again measured (Yadav et al. 2012). The formula developed by Ghoulam et al. (2002) was used to compute the electrolyte leakage. The percentage of electrolyte leakage is calculated using the equation (Eq. 1):
Leaf relative water content (RWC) was assessed on fully enlarged leaves from five plants per replicate. To determine RWC, five leaves were pulled from each replication and immediately weighed for fresh weight (FW). They were submerged in distilled water within a falcon tube for a duration of 24 h to regain turgor, and then, their turgor weight (TW) was detected. The samples were subjected to drying process at a temperature of 70 °C for 48 h to determine the dry weight (DW). The leaf RWC was calculated using the formula described by Ghoulam et al. (2002).
The turgid loss (TL) was measured following using the method (Turner 1986).
Statistical Analysis
The data were analyzed using the MSTAT-C computer program (Michigan State University v.2.10) in a completely randomized design with four replicates using a two-factor factorial. Data given in percentages were subjected to an arcsine transformation before statistical analysis. The means were compared using Duncan’s multiple range test at the p < 0.05.
Results
Significant differences among sugar beet cultivars were determined for damage percentage at all developmental stages. At the V1.1 stage, Orthega had the highest damage percentage of 56.8%, followed by Isabella (48.1%), Ernestina (35.3%), Smılodon (33.2%), and Serenada (30.9%) (Table 2). Other cultivars’ damage percentages were less than 30%, with Tuna (13.7%) and Taurus (14.8%) having the lowest percentages. At the V2.1 stage, Taurus (74.6%) and Mohican (42.9%) were seriously affected by frost stress, whereas Ernestina, Isabella, Serenada, Kuno, and Smılodon were unaffected. At V3.1, only two cultivars, Smılodon (25.6%) and Taurus (11.1%), were slightly harmed. In general, sugar beet plantlets gained tolerance against frost when the growth stage progressed, with mean damage percentages decreasing from 29.7% at V1.1 to 3.6% at V3.1.
The leaf chlorophyll content of sugar beet cultivars was significantly changed following frost treatment, and their responses were varied with growth stages. Chlorophyll content could not be measured at the V1.1 stage, because they did not have true leaves. However, in V2.1, leaf chlorophyll content ranged from 21.3 to 38.5 SPAD in plants subjected to frost stress and 28.6–38.9 SPAD in the control. The chlorophyll content of the cultivars showed a significant variation, while there was no statistical difference among the cultivars Orthega, Serenada, and Smılodon. After frost treatment, the highest leaf chlorophyll content was detected in Orthega, without significant differences with Ernestina and Serenada. At the V3.1 stage, Ernestina, Isabella, Orthega, Serenada, and Kuno had the highest chlorophyll content before and after frost damage. Differences in chlorophyll content between control and frost-stressed plants showed that the earlier stage of growth of sugar beet cultivars was much more sensitive to frost stress (Table 3).
At V2.1 stage, frost-stressed Mohican, Taurus, Tuna, Rodeo, and Smılodon plants had lower leaf temperatures than control plants, with these cultivars having the lowest values (Table 4). The lowest leaf temperatures were recorded in Taurus, Tuna, and Mohican, both in control and frost stress conditions. The mean leaf temperature decreased following frost stress but did not alter at the V3.1 stage. Frost is likely to have a less severe effect if the development stage is delayed. Rodeo and Smılodon (26.9 °C) in the control group had the greatest leaf surface temperatures at V3.1 stage, whereas Smılodon (20.5 °C) in the frost-stressed plants had the lowest leaf surface temperatures. At the V3.1 growth stage, Mohican and Tuna had the lowest mean leaf surface temperature, just as they did at the V2.1 growth stage. In addition, all cultivars in the control group had leaf surface temperatures exceeding 22 °C at both development stages (Table 4).
The electrolyte leakage increased from 15.6% in control to 52.6% in frost stress at the stage of V2.1 (Table 5). There were no significant differences between sugar beet cultivars in the control, while electrolyte leakage of frost-treated plants ranged from 14.1 to 95.4%. Frost stress had a significant impact on sugar beet cultivars Taurus, Tuna, and Mohican, which had electrolyte leakage higher than 90%. Isabella, Orthega, and Ernestina had the lowest electrolyte leakage, indicating that they were less influenced by frost stress than the other cultivars. However, at the later development stage V3.1, the leaves of Ernestina (71.2%) leaked much more electrolyte than the others, followed by Kuno (13.0%), Serenada (12.6%), Taurus (12.6%), and Orthega (11.8%).
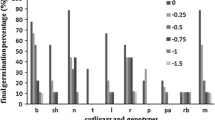
The mean RWC of the cultivars enhanced from 69.4% in control plants to 84.3% in frost-stressed plants at the V2.1 stage. Rodeo, Smılodon, and Serenada had the least relative water content in control. The RWC of frost-treated plants ranged between 77.4 and 95.9% (Table 6). The highest relative water contents in frost-stressed plants are observed in Kuno (95.9%), followed by Mohican (91.7%) and Taurus (91.4%). Variation between control and frost-stressed plants was positive, and relative water content was higher in stressed plants (Fig. 1). At the V3.1 stage, sugar beet cultivars did not show any significant differences in terms of control or frost stress.
Frost stress caused a reduction in the loss of turgidity in sugar beet cultivars at the V2.1, and it declined from 28.6 to 16.0% (Table 7). The largest decline was observed in Kuno (from 26.9 to 5.5%), with a variation value of − 21.3 (Fig. 1). At the V3.1 stage, turgid loss was measured to be lower than at V2.1. Rodeo and Smılodon had the least turgor loss in control and were easily distinguished from the other sugar beet cultivars.
The correlation coefficients between damage percentage and the investigated characteristics at V2.1 and V3.1 stages are presented in Table 8 and 9, respectively. All the correlations in the frost-stressed were found to be significant at V2.1 stage. Correlation coefficients showed that a negative correlation was recorded between DP and CHL with ST, and a positive correlation was recorded between DP and EL with RWC. Only a significant correlation was observed between DP and ST for all physiological traits in the control group. Furthermore, CHL was negatively significant correlated with RWC in both groups. DP was negatively significant correlated with CHL and ST and positively significant correlated with EL in frost-stressed at V3.1 stage. In the control group, DP was positively significant correlated with RWC, EL, and ST. At both V2.1 and V3.1 stages, the correlation of DP with EL showed similar results.
Discussion
Frost damage is one of the most dangerous abiotic stress factors in sugar beet cultivation in Türkiye. In this study, ten sugar beet cultivars commonly preferred by farmers in the Central Anatolia region and registered by different producers were tested for frost tolerance at the V1.1, V2.1, and V3.1 stages. The results showed that frost tolerance changed with the growth stages of the plants. The most sensitive stage of sugar beet to frost stress was the V1.1 stage, where about 30% of plants were damaged, with genotypic differences. In general, later growth stages resulted in increased tolerance. However, there were significant differences among sugar beet cultivars. Tuna and Taurus were the least damaged cultivars by frost at the V1.1 stage, while Orthega was the most tolerant cultivar at the V2.1 stage. At the V3.1 stage, no frost damage was observed in all sugar beet cultivars except for Smılodon and Taurus. In the previous research, Nezami et al. (2011) determined that the critical low temperature for the survival of sugar beet was below − 8 °C, while Jalilian et al. (2009) reported that temperatures of − 2 and − 4 °C damaged sugar beet seedlings. Recently, Moliterni et al. (2015) indicated that sugar beet seedlings were severely damaged at temperatures below 0 °C. In our study, several combinations of temperature and duration were first tested, and then, the combination of − 3 °C and 2 h was suitable for separating genotypic variation in sugar beet. Earlier stages of the growth of sugar beet and prolonged exposure to frost temperatures of − 5 °C caused significant reductions in vitality. Similar results were observed in potato by Angmo et al. (2023) who determined > 30% foliage damage after frost, but they informed that potato plants had the ability to resume vegetative growth after the endurance of frost. In this study, physiological characteristics were not measured at the V1.1 stage because the sugar beet plants had only two cotyledonary leaves. In sugar beet plants at stages V2.1 and V.3.1 subjected to frost temperature, the chlorophyll content increased in Kuno, Taurus, Tuna, and Mohican cultivars, suggesting that these cultivars were more susceptible to freezing temperatures. Similarly, Allinne et al. (2009) reported that cold temperatures during the early developmental stages of sunflower plants adversely affected chlorophyll content; Wijewardana et al. (2016) stated that the total chlorophyll content (a + b) of corn hybrids was lower in plants grown at low temperatures than in plants grown at moderately low or optimum temperatures. Jalilian et al. (2009) indicated that leaf chlorophyll fluorescence of sugar beet cultivars diminished with decreasing temperature, and sugar beet cultivars at the two-leaf stage were more sensitive than the four-leaf stage. Nezami et al. (2011) found that significant decreases in SPAD values of sugar beet cultivars at 4–5 leaf stages at temperatures lower than − 2 °C. On the other hand, leaf surface temperature increased in sugar beet cultivars subjected to frost stress at the V2.1 stage, it is not a clear clue for evaluating frost tolerance among sugar beet cultivars. This result is confirmed by the findings of Perry (1986) who reported that the surface temperature of plants in radiative night frost conditions might be lower than the air temperature by 1.6–2.7 °C. Contrarily, the lowest electrolyte leakage was measured in Tuna cultivar at both seedling stages, and higher electrolyte leakage was observed at the V2.1 stage. This finding is confirmed by Dix et al. (1994) who reported that the cold temperature tolerance of sugar beet cultivars could be evaluated by electrolyte leakage. Nezami et al. (2013) found a negative correlation between electrolyte leakage and survival percentage in sugar beet. Increases in leaf electrolyte leakage occurred with increasing freezing temperature and duration applied to sunflowers in the early stage by Hejnak et al. (2014) and Hnilickova et al. (2017). Besides, Angmo et al. (2023) found a significant increase in cell membrane injury in potato genotypes due to freezing. At the V2.1 stage, leaf relative water content of all cultivars under freezing temperature enhanced compared to the control group. Especially in Kuno, Mohican, and Taurus cultivars, leaf relative water content was above 90% and loss of turgidity was below 10%. The results show that these cultivars were more affected by freezing temperatures than other cultivars. Our results were supported by the findings of Srivastava et al. (1988), who determined that a significant reduction in leaf relative water content was an indicator of plant loss of turgidity. Dhanda and Sethi (2002) informed that leaf relative water content was the balance between transpiration and water supplied to the leaf, the more water the plant can take, the more it can save itself from stress. Hejnak et al. (2014) found that osmotic potential decreased after cold application to sunflower in the early vegetative stage, while no significant changes in with cold application to sunflower in the 6–8 leaf stage were recorded by Hnilickova et al. (2017). Also, Wijewardana et al. (2016) stated that 11 hybrids had more relative damage than the average value of 6%, revealing that such hybrids can preserve membrane stability during cold stress to maintain normal physiological metabolism.
Conclusion
In the Central Anatolia region, farmers have to delay sugar beet sowing until the 1st week of May to avoid late spring frost damage. Therefore, it is very important to select frost-tolerant sugar beet cultivars to permit farmers to early planting. Our results revealed that sugar beet gained tolerance against frost when the growing stages progressed. Also, there are significant variations among sugar beet cultivars for frost resistance. Among the investigated cultivars, Tuna, Taurus, and Rodeo were more tolerant to frost at V1.1, while Isabella, Ernestina, and Orthega at V2.1 than the other cultivars because the tolerant ones had the minimum damage rate and electrolyte leakage. Electrolyte leakage should be considered as a useful criterion for selecting frost-tolerant plants along with leaf temperature, chlorophyll content, and RWC in sugar beet.
References
Allinne, C., P. Maury, A. Sarrafi, and P. Grieu. 2009. Genetic control of physiological traits associated to low temperature growth in sunflower under early sowing conditions. Plant Science 177 (4): 349–359. https://doi.org/10.1016/j.plantsci.2009.07.002.
Angmo, D., S.P. Sharma, A. Kalia, N.S. Brar, and V. Bhardwaj. 2023. Effect of cold stress on field performance, chlorophyll fluorescence, electrolyte leakage and leaf gas exchange parameters of potato (Solanum tuberosum L.) genotypes. Potato Research 66(3):641–661. https://doi.org/10.1007/s11540-022-09593-6
Anonymous. 2023. https://biruni.tuik.gov.tr/medas/?locale=tr. Accessed September 10, 2023
Dhanda, S.S., and G.S. Sethi. 2002. Tolerance to drought stress among selected Indian wheat cultivars. The Journal of Agricultural Science 139 (3): 319. https://doi.org/10.1017/S0021859602002526.
Dix, P.J., I. Finch, and J.I. Burke. 1994. Genotypic differences in cold tolerance are masked by high sucrose and cytokinin in shoot cultures of sugar beet. Plant Cell, Tissue Organ Culture 36: 285–290.
Doğru, A. 2019. Low temperature stress and cold acclimation in plants. Süleyman Demirel University Journal of Natural and Applied Sciences 2 (1): 45–52.
Ghoulam, C., A. Foursy, and K. Fares. 2002. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environmental and Experimental Botany 47: 39–50. https://doi.org/10.1016/S0098-8472(01)00109-5.
Hejnak, V., L. Nemcova, M. Matejovic, J. Martinkova, F. Hnilicka, M. Skalicky, and F. Grieu. 2014. Physiological responses as influenced by night freeze stress at the beginning of vegetative growth of sunflower. Research on Crops 15 (2): 473–480. https://doi.org/10.5958/2348-7542.2014.00140.5.
Hnilickova, H., H. Vaclav, N. Lenka, M. Jaroslava, S. Milan, H. Frantisek, and G. Philippe. 2017. The effect of freezing temperature on physiological traits in sunflower. Plant, Soil and Environment 63(8):375–380. https://doi.org/10.17221/214/2017-PSE
Holen, C.D., and A.G. Dexter. 1996. A growing degree day equation for early sugar beet leaf stages. Sugarbeet Research and Extension Reports 27: 152–157.
Jalilian, A., D. Mazaheri, R. Tavakol Afshari, M. Abd Elahian Noughabi, H. Rahimian Mashhadi, and A. Ahmadi. 2009. Effect of freezing damage at seedling stage in different sugar beet cultivars. Iranian Journal of Crop Sciences 10 (4): 400–415.
Kirchhoff, M., A. Svirshchevskaya, C. Hoffman, A. Schechert, C. Jung, and F. Kopisch-Obuch. 2012. High degree of genetic variation of winter hardiness in a panel of Beta vulgaris L. Crop Science 52: 179–188. https://doi.org/10.2135/cropsci2011.04.0185.
Koçak, Ş.F., E.G. Kulan, and M.D. Kaya. 2019. The effects of harvest date and field storage duration on yield and sugar rate of sugar beet. COMU Journal of Agriculture Faculty 7(2): 313–321. https://doi.org/10.33202/comuagri.565631
Moliterni, V.M.C., R. Paris, C. Onofri, L. Orru, L. Cattivelli, D. Pacifico, C. Avanzato, A. Ferrarini, M. Delledonne, and G. Mandolino. 2015. Early transcriptional changes in Beta vulgaris in response to low temperature. Planta 242: 187–201. https://doi.org/10.1007/s00425-015-2299-z.
Nayyar, H., K. Chander, S. Kumar, and T. Bains. 2005. Glycine betaine mitigates cold stress damage in chickpea. Agronomy for Sustainable Development 25 (3): 381–388. https://doi.org/10.1051/agro:2005033.
Nezami, A., K. Hajmohammadnia Ghalibaf, and A. Kamandi. 2011. Evaluation of freezing tolerance of sugar beet (Beta vulgaris L.) cultivars under controlled conditions. Environmental Stress in Crop Sciences 3(2):177–187. https://doi.org/10.22077/escs.2011.93
Nezami, A., H. Khazaei, M. Dashti, H.R. Mehrabadi, E. Eyshi Rezaee, and M. Ahmadi. 2013. Evaluation of morpho-physiological indices in autumn sugar beet (Beta vulgaris L.) cultivars under freezing stress at seedling stage. Journal of Sugar Beet 29(1):15–31. https://doi.org/10.22092/jsb.2013.1301
Perry, K.B. 1986. FROSTPRO, a model of overhead irrigation rates for frost/freeze protection of apple orchards. HortScience 21 (4): 1060–1061.
Reyes, B.G., and J.M. McGrath. 2003. Cultivar-specific seedling vigor and expression of a putative oxalate oxidase germin-like protein in sugar beet (Beta vulgaris L.). Theoretical and Applied Genetics 107(1):54–61. https://doi.org/10.1007/s00122-003-1229-9
Srivastava, J.P., S.C. Gupta, P. Lal, R.N. Muralia, and A. Kumar. 1988. Effect of salt stress on physiological and biochemical parameters of wheat. Annals of Arid Zone 27: 197–204.
Stevanato, P. 2005. Resistance to abiotic stresses. In Genetics and Breeding of Sugar Beet, ed. E. Biancardi, L.G. Campbell, G.N. Skaracis, and M. De Biaggi, 116–119. USA: Science Publisher Inc.
Turner, N.C. 1986. Crop water deficit: a decade of progress. Advances in Agronomy 39: 1–51.
Wijewardana, C., W.B. Henry, M.W. Hock, and K.R. Reddy. 2016. Growth and physiological trait variation among corn hybrids for cold tolerance. Canadian Journal of Plant Science 96 (4): 639–656. https://doi.org/10.1139/cjps-2015-028.
Yadav, N.S., P.S. Shukla, A. Jha, P.K. Agarwal, and B. Jha. 2012. The SbS0S1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biology 12: 188. https://doi.org/10.1186/1471-2229-12-188.
Acknowledgements
The author thanks the Scientific Research Projects Coordinatorship (BAP) of Eskişehir Osmangazi University under grant number 2020-23048, and Dr. N. Ergin, and Ph.D. students M.F. Kaya for their kind help.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kulan, E.G., Kaya, M.D. Evaluation of Frost Tolerance in Sugar Beet Cultivars During Early Growth Stages. Sugar Tech (2024). https://doi.org/10.1007/s12355-024-01398-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12355-024-01398-w