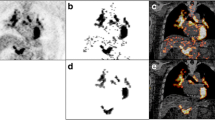
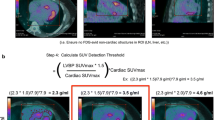
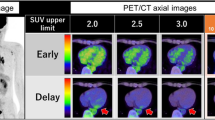
Abstract
Background
Quantitative assessment of cardiac hypermetabolism from 18Flourodeoxy glucose (FDG) positron emission tomography (PET) may improve diagnosis of cardiac sarcoidosis (CS). We assessed different approaches for quantification of cardiac hypermetabolism and perfusion in patients with suspected CS.
Methods and results
Consecutive patients undergoing 18FDG PET assessment for possible CS between January 2014 and March 2019 were included. Cardiac hypermetabolism was quantified using maximal standardized uptake value (SUVMAX), cardiometabolic activity (CMA) and volume of inflammation, using relative thresholds (1.3× and 1.5× left ventricular blood pool [LVBP] activity), and absolute thresholds (SUVMAX > 2.7 and 4.1). Diagnosis of CS was established using the Japanese Ministry of Health and Wellness criteria. In total, 69 patients were studied, with definite or possible CS in 29(42.0%) patients. CMA above 1.5× LVBP SUVMAX had the highest area under the receiver operating characteristic curve (AUC 0.92). Quantitative parameters using relative thresholds had higher AUC compared to absolute thresholds (p < 0.01). Interobserver variability was low for CMA, with excellent agreement regarding absence of activity (Kappa 0.970).
Conclusions
Quantitation with scan-specific thresholds has superior diagnostic accuracy compared to absolute thresholds. Based on the potential clinical benefit, programs should consider quantification of cardiac hypermetabolism when interpreting 18F-FDG PET studies for CS.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- CS:
-
Cardiac sarcoidosis
- FDG:
-
Fluorodeoxyglucose
- JMHW:
-
Japanese Ministry of Health and Welfare
- LVEF:
-
Left ventricular ejection fraction
- LVBP:
-
Left ventricular blood pool
- PET:
-
Positron emission tomography
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- SRS:
-
Summed rest score
- SUVMAX. :
-
Maximal standardized uptake value
- TPD:
-
Total perfusion deficit
- VOI:
-
Volume of inflammation
References
Hamzeh N, Steckman DA, Sauer WH, Judson MA. Pathophysiology and clinical management of cardiac sarcoidosis. Nature Rev Cardiol. 2015;12:278–88.
Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis—state of the art review. Cardiovasc Diagn Ther. 2016;6:50–63.
Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336–46.
Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578–83.
Sadek MM, Yung D, Birnie DH, Beanlands RS, Nery PB. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–41.
Popovtzer M, Robinette J, Halgrimson C, Starzl T. Acute effect of prednisolone on renal handling of sodium. Am J Physiol. 1973;224:651–8.
Akaike G, Itani M, Shah H, Ahuja J, Gunes BY, Assaker R, et al. PET/CT in the diagnosis and workup of sarcoidosis: focus on atypical manifestations. RadioGraphics. 2018;38:1536–49.
Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–6.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36.
Ishida Y, Yoshinaga K, Miyagawa M, Moroi M, Kondoh C, Kiso K, et al. Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis. Ann Nucl Med. 2014;28:393–403.
Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21:925–39.
Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–74.
Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart rhythm. 2012;9:884–91.
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–226.
Dekemp RA, Declerck J, Klein R, Pan XB, Nakazato R, Tonge C, et al. Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J Nucl Med. 2013;54:571–7.
Nakazato R, Berman DS, Dey D, Le Meunier L, Hayes SW, Fermin JS, et al. Automated quantitative Rb-82 3D PET/CT myocardial perfusion imaging: normal limits and correlation with invasive coronary angiography. J Nucl Cardiol. 2012;19:265–76.
Slart RHJ, Glaudemans AW, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, et al. A joint procedural position statement on imaging in cardiac sarcoidosis. EHJ Cardiovasc Imaging. 2017;18:1073–89.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5.
Lebasnier A, Legallois D, Bienvenu B, Bergot E, Desmonts C, Zalcman G, et al. Diagnostic value of quantitative assessment of cardiac (18)F-fluoro-2-deoxyglucose uptake in suspected cardiac sarcoidosis. Ann Nucl Med. 2018;32:319–27.
Varghese M, Miller E. Quantitative interpretation of FDG PET for cardiac sarcoidosis reclassifies visually interpreted studies and potentially reduces unnecessary downstream interventions. J Nucl Med. 2017;58:512.
Ohira H, Ardle BM, deKemp RA, Nery P, Juneau D, Renaud JM, et al. Inter- and intraobserver agreement of (18)F-FDG PET/CT image interpretation in patients referred for assessment of cardiac sarcoidosis. J Nucl Med. 2017;58:1324–9.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016;6:52.
Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017;24:413–24.
Ardle BAM, Birnie DH, Klein R, Kemp RAD, Leung E, Renaud J, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 18F-fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6:617–26.
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm. 2014;11:1304–23.
Disclosures
The work was supported in part by the Miriam and Sheldon Adelson Medical Research Foundation. Drs. Berman, and Slomka participate in software royalties for QPS software at Cedars-Sinai Medical Center. Robert Miller, Sebastien Cadet, Payam Pournazari, Adele Pope, Evan Kransdorf, Michele A Hamilton, Jignesh Patel, Sean Hayes, John Friedman, Louise Thomson, and Balaji Tamarappoo have no relevant disclosures.
Funding
This research was supported in part by grant R01HL089765 from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH) (PI: Piotr Slomka). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miller, R.J.H., Cadet, S., Pournazari, P. et al. Quantitative Assessment of Cardiac Hypermetabolism and Perfusion for Diagnosis of Cardiac Sarcoidosis. J. Nucl. Cardiol. 29, 86–96 (2022). https://doi.org/10.1007/s12350-020-02201-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02201-5