Abstract
Introduction
In contrast to the antihypertensive effect of esaxerenone, there is little evidence of its cardioprotective effect. We investigated the efficacy and safety of esaxerenone in patients with uncontrolled hypertension and left ventricular hypertrophy taking a renin-angiotensin system inhibitor (RASi) or calcium-channel blocker (CCB).
Methods
This was a multicenter, open-label, exploratory study with a 24-week treatment period. Esaxerenone was orally administered at an initial dose of 2.5 mg/day (maximum dose: 5 mg/day). The primary endpoints were the change in morning home systolic blood pressure (BP)/diastolic BP and change and percentage change in left ventricular mass index (LVMI) from baseline to end of treatment (EOT). Key secondary endpoints included change from baseline in bedtime home and office BP, achievement rate of target BP, and safety.
Results
In total, 60 patients were enrolled. Morning home systolic/diastolic BP was significantly decreased from baseline to EOT in the total population (− 11.5/ − 4.7 mmHg, p < 0.001) and in both the RASi and CCB subcohorts (all p < 0.01). Significant reductions in bedtime home and office BP were shown in the total population and both subcohorts. LVMI was also significantly decreased from baseline to EOT in the total population (− 9.9 g/m2, − 8.5%, both p < 0.001) and both subcohorts (all p < 0.05). The incidences of treatment-emergent adverse events (TEAEs) and drug-related TEAEs were 35.0% and 3.3%, respectively; most were mild or moderate. No new safety concerns were identified.
Conclusion
Esaxerenone showed favorable antihypertensive and cardioprotective effects and safety in hypertensive patients with cardiac hypertrophy.
Trial Registration
Japan Registry of Clinical Trials (jRCTs071190043).
Similar content being viewed by others
Why carry out this study? |
Attenuation of cardiac hypertrophy with sustained antihypertensive treatment is important to delay the progression to heart failure; however, it is difficult to achieve the target blood pressure with existing antihypertensive drug monotherapy such as renin-angiotensin system inhibitors (RASis) and calcium-channel blockers (CCBs). |
Esaxerenone is a selective nonsteroidal mineralocorticoid receptor blocker that has shown favorable antihypertensive effects in hypertensive patients with various background characteristics, but there is no clinical evidence in hypertensive patients with cardiac hypertrophy. |
We investigated the antihypertensive and left ventricular hypertrophy-regressive effects and the safety of esaxerenone in hypertensive patients with cardiac hypertrophy who had inadequate responses to a RASi or CCB. |
What was learned from the study? |
Esaxerenone demonstrated consistent blood pressure-lowering and left ventricular hypertrophy-regressive effects in hypertensive patients with cardiac hypertrophy regardless of the concomitant use of a RASi or CCB. |
Esaxerenone may be a suitable antihypertensive treatment option accompanied by cardioprotective effects for hypertensive patients with left ventricular hypertrophy. |
Furthermore, esaxerenone can be used safely in these patients without clinically relevant serum potassium elevation and reduction in creatinine-based estimated glomerular filtration rate. |
Introduction
Left ventricular hypertrophy (LVH) is associated with a high risk of major cardiovascular events, including ischemic heart disease, fatal arrhythmia, and sudden death [1]. Hypertension is an independent risk factor for the development of LVH, and up to 60% of patients with hypertension have concomitant LVH [2]. Reversal of LVH with antihypertensive drugs significantly reduces the risk for cardiovascular events [3]. Cardiac hypertrophy is a prognostic factor in patients with hypertension, and attenuation of hypertrophy with sustained antihypertensive treatment is important to delay the progression to heart failure [4, 5].
In the SPRINT trial, intensive blood pressure (BP) reduction (systolic BP [SBP] < 120 mmHg) reduced the occurrence of new LVH compared with standard BP reduction (target SBP < 140 mmHg) and also increased the rate of LVH regression in patients with a history of LVH [6]. Thus, antihypertensive therapy aimed at achieving target BP leads to regression of the left ventricular mass (LVM) [7]. Among antihypertensive drug classes, renin-angiotensin system inhibitors (RASis) and calcium-channel blockers (CCBs) have been found to be the most effective in the regression of LVH (LVM index [LVMI] reduction: diuretics by 8%; β-blockers, 6%; CCBs, 11%; angiotensin-converting enzyme [ACE] inhibitors, 10%; angiotensin-receptor blockers [ARBs], 13%) [8]; as such, RASis and CCBs are recommended as the first-line treatment option for hypertension complicated by cardiac hypertrophy in The Japanese Society of Hypertension (JSH) Guidelines for the Management of Hypertension [9]. However, evidence on LVH regression with concomitant use of antihypertensive drugs is scarce, and there is no mention of concomitant use of antihypertensive drugs, including the combination of RASi and CCB, in the JSH guideline [9]. Among Japanese, US, and European guidelines, only the European guideline recommends the use of RASi in combination with CCB or diuretics based on the results of a meta-analysis that showed no significant difference in heart failure risk reduction among antihypertensive drug classes, although only CCBs were inferior in preventing heart failure [4, 5, 9, 10]. Given this background, it is important to present new treatment options for hypertensive patients with cardiac hypertrophy other than existing antihypertensive drugs and to show the effect of combining the new treatment option with RASi or CCBs.
Evidence shows that blockade of mineralocorticoid receptors (MRs) is beneficial in hypertensive patients with different characteristics, regardless of their actual peripheral aldosterone levels [11,12,13]. Furthermore, the steroidal MR blockers (MRBs) spironolactone and eplerenone have been demonstrated to contribute to cardioprotection in cardiac hypertrophy and heart failure beyond their antihypertensive effects [14,15,16,17]. Therefore, MRBs are recognized as antihypertensive agents with cardioprotective properties. However, existing steroidal MRBs have some limitations, including hormone-related adverse effects such as gynecomastia and menstrual abnormalities with spironolactone [18, 19]. Furthermore, eplerenone is contraindicated in patients with hypertension with moderate or severe renal impairment and in those with diabetes mellitus-associated albuminuria because of the risk of hyperkalemia [20, 21].
Esaxerenone, a selective nonsteroidal MRB, has favorable antihypertensive effects in patients with hypertension with various background characteristics [22,23,24,25,26,27,28,29,30] and has good renoprotective effects including reduction and remission of albuminuria in hypertensive patients with diabetic kidney disease [25, 26, 28,29,30,31]. Esaxerenone is also expected to exert cardioprotective effects in terms of regression of cardiac hypertrophy via its mechanism of action [32]. To date, however, there is insufficient clinical evidence to support its antihypertensive and cardioprotective effects in hypertensive patients with cardiac disease, although it has been validated in a few nonclinical and retrospective clinical studies [33,34,35,36,37].
In the present study, we investigated the antihypertensive and LVH-regressive effects of esaxerenone and its safety in hypertensive patients with cardiac hypertrophy who had an inadequate response to RASis or CCBs.
Methods
Study Design
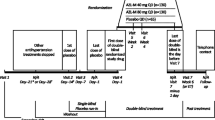
This was a multicenter, open-label, prospective, exploratory interventional study conducted from June 2020 to March 2022 at 20 sites in Japan. The list of all participating institutions and ESES-LVH investigators is shown in Table S1 in the electronic supplementary material. This study had a 4-week run-in period and a 24-week treatment period (see Fig. S1 in the electronic supplementary material). Details of the study design have been reported previously [38].
The protocol was approved by the Kumamoto University Certified Clinical Research Review Board (CRB7200002) and prospectively registered with the Japan Registry of Clinical Trials (jRCTs071190043; https://jrct.niph.go.jp/en-latest-detail/jRCTs071190043). The study was conducted in accordance with the Declaration of Helsinki and the Clinical Trials Act in Japan. All the study participants provided written informed consent before enrollment.
Study Patients
The study included patients: aged ≥ 20 years who had been taking a RASi or a CCB at a fixed dosage regimen from 28 days or earlier before the start of esaxerenone administration; with a mean sitting home SBP of 135 to ≤ 159 mmHg and/or diastolic BP (DBP) of 85 to ≤ 99 mmHg using an upper arm cuff sphygmomanometer in the past 5 days or more prior to the registration date and within 14 days before the start of esaxerenone administration; and with a diagnosis of LVH meeting any of the following criteria: thickening of the left ventricular posterior wall or intraventricular septal wall of ≥ 12 mm on echocardiogram, LVH with Sv1 + Rv5 ≥ 35 mm on electrocardiogram, and LVMI ≥ 125 g/m2 for men and ≥ 110 g/m2 for women. Key exclusion criteria were as follows: patients with secondary or malignant hypertension, type 1 diabetes mellitus, complication or history of orthostatic hypotension or cerebrocardiovascular disease and those with symptoms or contraindications for treatment with esaxerenone as indicated in the esaxerenone package insert such as hyperkalemia, serum potassium level > 5.0 mEq/l, or severe renal impairment defined as a creatinine-based estimated glomerular filtration rate (eGFRcreat) < 30 ml/min/1.73 m2 [39]. Of note, according to 2019 JSH Guidelines for the Management of Hypertension, target BP levels should be achieved promptly (within several weeks) in high-risk patients, such as those with grade III hypertension (defined as home SBP ≥ 160 mmHg and/or DBP ≥ 100 mmHg) and multiple risk factors [9]. Because hypertensive patients with LVH, the subjects of this study, are considered high-risk patients, patients with grade III hypertension were excluded from this study for ethical reasons. Detailed inclusion and exclusion criteria are shown in Table S2 in the electronic supplementary material.
Drug Intervention
Esaxerenone was orally administered once daily at an initial dose of 2.5 mg/day. If the efficacy was inadequate, the dose could be titrated up to 5 mg/day from Week 4 onwards based on patients’ BP and serum potassium level monitoring. In patients with moderate renal impairment and/or diabetes mellitus with microalbuminuria or proteinuria, esaxerenone was started at 1.25 mg/day and increased to 2.5 mg/day from Week 4 onwards depending on their BP and serum potassium level. If the efficacy was inadequate, the dose could be titrated up to 5 mg/day at Week 8. Dose reductions were considered based on the serum potassium levels, the criteria for which have been described previously [38]. During the study, no changes were made in the dosage regimen of basal antihypertensive medications (RASi or CCB). The following concomitant drugs were prohibited from 4 weeks prior to starting treatment to the end of treatment (EOT) or until discontinuation: antihypertensive and antianginal drugs (e.g., α-blockers, β-blockers, αβ-blockers, other sympatholytic agents, vasodilators, or renin inhibitors), diuretics (e.g., thiazide, thiazide-like, loop, or potassium-sparing diuretics), aldosterone antagonists, angiotensin receptor-neprilysin inhibitors, potassium preparations, serum potassium suppressants, and hyperkalemia ameliorants.
Measurement of BP
Home BP was self-measured twice daily (in the morning and at bedtime) as much as possible throughout the study period, including the last 5–7 days before starting esaxerenone administration. Patients used the same upper arm cuff sphygmomanometer throughout the study period. The average value of two-time BP measurements at each timepoint was recorded. Morning home BP was measured after urinating within 1 h after waking up and before breakfast, medication, and caffeine intake. Bedtime home BP was measured before bedtime and at least 1 h after bathing, drinking, or caffeine intake. Office BP was measured twice at each visit (at baseline; 4, 12, and 24 weeks; and at discontinuation), and the average of two measurements was recorded. Office BP was measured after at least 5 min of rest in a sitting position and at intervals of at least 3 h after meals. BP measurements at Week 8 were used to determine whether the esaxerenone dose should be increased for patients who had the dose increased from 1.25 mg at Week 4.
Transthoracic Echocardiography
Details of the methodology for echocardiograms have been reported previously [38]. Transthoracic echocardiography measurements were performed by sonographers at each medical institution at baseline, 12 weeks, 24 weeks, and discontinuation and evaluated by one independent blinded experienced observer in the Echocardiographic Assessment Committee. To reliably identify patients with LVH, the Echocardiographic Assessment Committee reviewed and validated all echocardiographic data and analyses at each medical institution. According to the recommendations of the American Society for Echocardiography and the European Association of Cardiovascular Imaging [40], LVM and LVMI were calculated by the following cube formula by two-dimensional echocardiography [41]: LVM (g) = 0.8 × 1.04 × [(interventricular septum thickness + left ventricular end-diastolic dimension [LVDd] + posterior wall thickness)3 − LV end-diastolic dimension (LVDd)3] + 0.6; body surface area (m2) = 0.008883 × height (cm)0.663 × weight (kg)0.444; and LVMI (g/m2) = LVM (g)/body surface area (m2). Left ventricular ejection fraction (LVEF), left atrial volume index (LAVI), the ratio between the E-wave velocity and A-wave velocity of the pulsed-wave Doppler mitral flow image (E/A), the ratio between E-wave velocity and the average early diastolic velocity of the lateral and septum at the mitral annulus level (E/e′) on tissue Doppler imaging, and tricuspid regurgitation velocity (TRV) were also evaluated.
Measurement of Other Biomarkers
Plasma aldosterone concentration (PAC), plasma renin activity (PRA), and serum N-terminal pro-brain natriuretic peptide (NT-proBNP) were measured at baseline, 12 weeks, 24 weeks, and discontinuation in a central measurement laboratory (LSI Medience Corp., Tokyo, Japan) at intervals of at least 3 h after meals and after resting in the supine position for at least 30 min. The eGFRcreat was calculated as follows: 194 × serum creatinine−1.094 × age−0.287, multiplied by 0.739 for women.
Outcomes
The primary efficacy endpoints were the change in morning home SBP/DBP and the change and percentage change in LVMI from baseline to the EOT. Key secondary efficacy endpoints were as follows: change in morning home SBP/DBP from baseline to Week 12, change in bedtime home and office SBP/DBP from baseline to Week 12 and EOT, time course change of home (morning and bedtime) and office SBP/DBP during the study period, achievement rate of target BP levels (SBP/DBP, < 135/85 mmHg for home BP and < 140/90 mmHg for office BP) at Week 24, change and percentage change in LVMI from baseline to Week 12, change in echocardiographic parameters from baseline to Week 12 and EOT (LVEF, LAVI, E/A, E/e′, and TRV), and change in blood biomarkers from baseline to Week 24 (PAC, PRA, and NT-proBNP).
The following safety endpoints were further evaluated: treatment-emergent adverse events (TEAEs), laboratory values, vital signs (body temperature and pulse rate), the incidences of serum potassium level ≥ 5.5 mEq/l and ≥ 6.0 mEq/l, and time course changes and changes from baseline in serum potassium and eGFRcreat.
Statistical Analysis
This was an exploratory study, and the number of cases was determined based on practicality; the analysis was not adjusted for multiplicity of testing caused by multiple evaluation groups and multiple timepoints. The target sample size was set at 120 patients in total (RASi, n = 60; CCB, n = 60). Based on the BP measurements in a long-term Phase 3 study of esaxerenone and the HONEST study [23, 42], it was assumed that the mean ± standard deviation (SD) change in morning home SBP/DBP of esaxerenone would be − 8.7/− 4.9 mmHg ± 19/11 mmHg. Based on a previous study [43], a mean ± SD change in LVMI of − 10 ± 22 g/m2 was also assumed. Under this assumption, with a two-sided significance level of 5% and 48 patients per cohort, the power would be ≥ 85% for both the change in morning home SBP/DBP and the change in LVMI. Therefore, the target number of patients for each cohort was set at 60 patients per cohort to account for exclusions from the analysis.
Analyses were conducted in the total population and stratified by baseline antihypertensive drugs (RASi and CCB subcohorts). No statistical comparisons were performed between subcohorts. Efficacy endpoints were evaluated in the full analysis set (FAS), defined as all patients from the safety analysis set who met the inclusion criteria and had at least one efficacy assessment during the treatment period. The per-protocol set (PPS) was defined as FAS patients who adhered to the esaxerenone package insert [39]. Mean ± SD changes in primary and secondary efficacy endpoints were calculated, along with the point estimate and 95% confidence interval (CI) for the percentage change, and comparisons were made using the paired t-test between baseline and each measurement point. EOT values were calculated by taking the average of measurements at the last two visits in the treatment period. The missing values at the EOT were imputed by the last observation carried forward method. The 95% CIs for achievement rate of target BP levels were calculated using the Clopper-Pearson method.
Safety endpoints were evaluated in the safety analysis set, defined as all patients who received at least one dose of esaxerenone. TEAEs were coded according to System Organ Class and Preferred Term using the Medical Dictionary for Regulatory Activities, version J.24.1.
Descriptive statistics were used to summarize patients’ demographic and clinical characteristics, including mean ± SD for continuous data and n (%) for categorical data. All tests were two sided with a significance level of 5%. All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ Characteristics
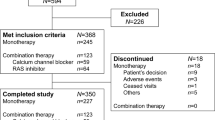
A total of 84 patients provided informed consent, of whom 60 met eligibility criteria and were enrolled in the study (21 in the RASi subcohort and 39 in the CCB subcohort). The initial target population was 120 patients, but due to the COVID-19 pandemic and torrential rain in Kumamoto Prefecture, enrollment was terminated at 60 patients. All 60 patients were included in the safety analysis set. Two patients were excluded for not meeting the inclusion criteria; finally, 58 patients (19 in the RASi and 39 in the CCB subcohort) were included in the FAS. The PPS included 46 patients (15 in the RASi and 31 in the CCB subcohort). A total of 57 patients (18 and 39 in each subcohort, respectively) completed the study.
Baseline demographic and clinical characteristics are summarized in Table 1. In the total population, most patients were male (70.7%), and the mean age was 64.8 years. The mean morning home SBP/DBP was 142.8/85.0 mmHg; bedtime home SBP/DBP, 141.0/81.9 mmHg; office SBP/DBP, 145.9/85.3 mmHg; mean eGFRcreat, 71.3 ml/min/1.73 m2; mean serum potassium level, 4.1 mEq/l; mean NT-proBNP, 191.8 pg/ml; mean LVMI, 118.1 g/m2; and mean LVEF, 62.8%. Overall, patient characteristics were well balanced between the RASi and CCB subcohorts, with notable differences in the proportion of male patients (84.2% and 64.1%) and the mean LVMI (127.9 and 113.9 g/m2) in the RASi and CCB subcohorts, respectively. In the total population, the dose of esaxerenone at EOT was 1.25 mg/day in five (8.6%) patients, 2.5 mg/day in 26 (44.8%) patients, and 5 mg/day in 27 (46.6%) patients, and these dose proportions were similar in both subcohorts.
BP Measurements
In the FAS, a statistically significant decrease in morning home BP levels from baseline to EOT was shown in the total population (− 11.5/− 4.7 mmHg, p < 0.001) and RASi and CCB subcohorts (− 11.6/ − 6.6 mmHg and − 11.5/ − 3.8 mmHg, respectively; all p < 0.01) (Fig. 1a and b, and Table S3 in the electronic supplementary material). Similar to the morning home BP, significant reductions were also shown in bedtime home SBP/DBP (all p < 0.01) and office SBP/DBP (all p < 0.01) from baseline to EOT in the total population and RASi and CCB subcohorts (Fig. 1c and d, and Table S3 in the electronic supplementary material). Also, at 12 weeks, morning and bedtime home and office BP measurements were significantly reduced compared to baseline in the total population and the RASi and CCB subcohorts (all p < 0.01) (Fig. 2, and Figs. S2 and S3, and Table S3 in the electronic supplementary material). In the PPS, each BP measurement showed a similar significant reduction (Table S4 in the electronic supplementary material).
Change from baseline in morning home BP level at EOT in the total population (a) and RASi or CCB subcohorts (b) and change from baseline in bedtime home BP level (c) and office BP level (d) at EOT in the total population and RASi or CCB subcohorts (full analysis set). Data are mean (95% confidence interval). **p < 0.01, ***p < 0.001 vs. baseline, paired t-test. BP blood pressure, CCB calcium channel blocker, DBP diastolic blood pressure, EOT end of treatment, RASi renin-angiotensin system inhibitor, SBP systolic blood pressure
Time course changes (a) and changes from baseline (b) in morning home BP throughout the study period in the total population and RASi or CCB subcohorts (full analysis set). Data are mean ± standard deviations. *p < 0.05, **p < 0.01, ***p < 0.001 vs. baseline, paired t-test. BP blood pressure, CCB calcium channel blocker, DBP diastolic blood pressure, EOT end of treatment, RASi renin–angiotensin system inhibitor, SBP systolic blood pressure
Time course changes in morning home BP, bedtime home BP, and office BP in the total population and RASi and CCB subcohorts are shown in Fig. 2, and Figs. S2 and S3 in the electronic supplementary material. In the total population, morning home and bedtime home BP significantly decreased starting at Week 2, continued decreasing up to Week 6, and thereafter remained constant until Week 24. Similar results were shown in the RASi and CCB subcohorts. Office BP significantly decreased up to Week 12 and thereafter remained constant until Week 24 in the total population and the RASi and CCB subcohorts.
The achievement rate of target morning home SBP/DBP level at Week 24 in the FAS was 56.9% in the total population, 63.2% in the RASi subcohort, and 53.8% in the CCB subcohort, and the achievement rates of bedtime home and office SBP/DBP levels also ranged from 50 to 70% in the total population and both subcohorts (Table S5 in the electronic supplementary material). Similar trends were observed in the PPS (Table S6 in the electronic supplementary material).
Echocardiographic Parameters
The changes and geometric percentage changes in LVMI from baseline to EOT in the FAS are shown in Table S7, and these results were similar in the PPS (Table S8 in the electronic supplementary material). A statistically significant reduction from baseline in LVMI at EOT was shown in the total population (mean change: − 9.9 g/m2, p < 0.001; Fig. 3a and Table S7 in the electronic supplementary material). Significant reductions were also shown in the RASi (− 13.9 g/m2, p < 0.01) and CCB (− 8.2 g/m2, p < 0.05) subcohorts (Fig. 3b and Table S7 in the electronic supplementary material). Similar trends were shown in the geometric percentage change from baseline in LVMI at EOT (total population: − 8.5%, p < 0.001; RASi subcohort: − 10.4%, p < 0.01; CCB subcohort: − 7.7%, p < 0.05) (Fig. 3c and Table S7 in the electronic supplementary material). Also, at 12 weeks, changes and percentage changes from baseline in LVMI significantly decreased from baseline in the total population and the RASi and CCB subcohorts (Fig. 3c and Table S7 in the electronic supplementary material).
Change from baseline in LVMI at EOT in the total population (a) and RASi or CCB subcohorts (b), time course geometric percentage change from baseline in LVMI in the total population and RASi or CCB subcohorts (c), and geometric percentage change from baseline in NT-proBNP at Week 24 (d) (full analysis set). Data are mean (95% confidence interval). *p < 0.05, **p < 0.01, ***p < 0.001 vs. baseline, paired t-test. CCB calcium channel blocker, EOT end of treatment, LVMI left ventricular mass index, NT-proBNP N-terminal pro-brain natriuretic peptide, RASi renin–angiotensin system inhibitor
Changes in other echocardiogram measurements from baseline to EOT in the FAS are shown in Table S9 (see the electronic supplementary material), and these results were similar in the PPS (Table S10 in the electronic supplementary material). An increasing trend in LVEF was shown in the total population and the RASi and CCB subcohorts, with a statistically significant increase shown only in the RASi cohort. The LAVI tended to decrease in the total population and the RASi and CCB subcohorts, but the change did not reach statistical significance. The E/A decreased significantly in the total population and the RASi subcohort, but the decrease did not reach statistical significance in the CCB subcohort. The E/e′ decreased significantly in the total population and the CCB subcohort, but the decrease did not reach statistical significance in the RASi subcohort. No clinically meaningful change was observed in the TRV.
Effects of Esaxerenone on Blood Biomarkers
Changes in biomarker data from baseline in the FAS are summarized in Table S11, and these results were similar in the PPS (Table S12 in the electronic supplementary material). NT-proBNP decreased significantly from baseline to Week 24 in the total population and CCB subcohort (geometric percentage change, − 13.9% and − 15.7%, respectively; both p < 0.05); in the RASi subcohort, the geometric percentage change decreased by − 9.3%, but the change was not statistically significant (Fig. 3d). PAC and PRA increased with esaxerenone administration from baseline to Weeks 12 and 24 in the total population (mean ± SD change: PAC, 77.7 ± 63.5 and 78.6 ± 60.0 pg/ml; PRA, 3.4 ± 4.7 and 3.1 ± 5.6 ng/ml/h, respectively). Similar increases were observed in the RASi and CCB subcohorts.
Safety
TEAEs are summarized in Table 2. The proportion of patients with at least one TEAE was 35.0%. Drug-related TEAEs occurred in two (3.3%) patients, of whom one (1.7%) discontinued the study treatment (blood potassium increased). Most TEAEs were mild or moderate. Serious TEAEs occurred in two (3.3%) patients, one with acute sinusitis and another with atrial fibrillation and diabetes mellitus, which were not related to esaxerenone treatment. Frequent TEAEs that occurred in ≥ 2 patients were arthralgia and nasopharyngitis (5.0% each), followed by blood potassium increased and liver disorder (3.3% each).
The eGFRcreat decreased up to 4 weeks and then was maintained up to 24 weeks (Fig. 4a). The mean change in eGFRcreat from baseline to Week 24 was − 6.9 ± 6.9 ml/min/1.73 m2 (Fig. S4a in the electronic supplementary material). This trend was similar in both the RASi and CCB subcohorts.
Time course changes in eGFRcreat (a) and serum potassium levels (b) throughout the study period in the total population and RASi or CCB subcohorts (safety analysis set). Data are mean ± standard deviations. CCB calcium channel blocker, eGFRcreat creatinine-based estimated glomerular filtration rate, RASi renin–angiotensin system inhibitor
Serum potassium levels increased over the first 2 weeks and then were maintained up to 24 weeks (Fig. 4b). The mean changes in serum potassium from baseline to Weeks 2 and 24 were 0.19 ± 0.33 and 0.23 ± 0.38 mEq/l, respectively (Fig. S4b in the electronic supplementary material), and the trend was similar in both the RASi and CCB subcohorts. The incidence of serum potassium level ≥ 5.5 mEq/l was 5.0% in the total population, occurring in three patients. This included one (4.8%) and two (5.1%) patients in the RASi and CCB subcohorts, respectively (Table S13 in the electronic supplementary material). No patients presented with serum potassium level ≥ 6.0 mEq/l.
Discussion
The ESES-LVH study evaluated the efficacy and safety of the nonsteroidal MRB esaxerenone in uncontrolled hypertensive patients with LVH taking a RASi or a CCB. Findings from this study add to the evidence of the cardioprotective effects of esaxerenone in patients with cardiac hypertrophy regardless of the concomitant use of RASi or CCB.
Esaxerenone significantly reduced the primary endpoint of morning home SBP/DBP from baseline to EOT in the total population (− 11.5/− 4.7 mmHg, p < 0.001) and in the RASi and CCB subcohorts (− 11.6/ − 6.6 mmHg and − 11.5/ − 3.8 mmHg, respectively; all p < 0.01). Similar significant reductions in the total population and both subcohorts were observed in bedtime home and office SBP/DBP, indicating that esaxerenone would exhibit a consistent antihypertensive effect in this patient population and that these effects would be independent of concomitant use of RASis or CCBs. The reduction in morning home SBP/DBP in this study was comparable to the EX-DKD study in hypertensive patients with diabetic kidney disease treated with a RASi alone or a RASi plus CCB (− 11.6/− 5.2 mmHg) [30]. In addition, the change in office SBP/DBP in this study (− 15.1/ − 6.5 mmHg) was also similar to that shown in phase 3 studies of esaxerenone in RASi-treated hypertensive patients with moderate renal dysfunction (− 17.8/ − 8.1 mmHg) [25] and with type 2 diabetes associated with microalbuminuria (− 13.7/− 6.2 mmHg) [26]. Together with the fact that the reduction in the office SBP/DBP at Week 12 in the long-term Phase 3 study in patients with essential hypertension (with RASi − 16.8/− 9.6 mmHg and with CCB − 14.8/− 8.2 mmHg) was also comparable to that of the ESES-LVH study [23], esaxerenone may have a favorable antihypertensive effect in hypertensive patients with various complications, including cardiac hypertrophy. Although this study did not include a placebo control, its antihypertensive effect has been already confirmed in two placebo-controlled clinical trials [28, 44]. Furthermore, as in previous clinical studies of esaxerenone and other MRBs [22, 23, 30, 45, 46], PAC and PRA, indicators of MR activity inhibition, increased with the BP reduction in this study. Based on these results, it was deduced that the placebo antihypertensive effect was not the main factor in the BP reduction.
Esaxerenone also significantly reduced the primary endpoint of LVMI from baseline to EOT in the total population (− 9.9 g/m2, p < 0.001) and in the RASi and CCB subcohorts (− 13.9 g/m2 and − 8.2 g/m2, respectively; all p < 0.05). Over the 24-week study period, the change and percentage change in LVMI significantly decreased from baseline in a time-dependent manner, with percentage change in LVMI of − 6.5%, − 9.1%, and − 8.5% (all p < 0.01) by Week 12, Week 24, and EOT, respectively. This similar time-dependent decline was also observed in both subcohorts. This reductive effect on LVMI is consistent with the result of a previous retrospective study, the only study to evaluate the cardioprotective effect of esaxerenone over 6 months using echocardiography in hypertensive patients with heart failure with preserved ejection fraction (HFpEF) [35]. LVH regression was also reported with the other steroidal MRBs. In a randomized placebo-controlled 4E-left ventricular hypertrophy study in patients with LVH and hypertension, 9-month treatment with eplerenone and eplerenone/enalapril significantly reduced LVM from baseline (− 14.5 g, − 27.2 g, respectively) [47]. This reduction with eplerenone/enalapril was comparable to the 24-week reduction in the RASi subcohort of this study (− 23.6 g). Spironolactone has also been shown to reduce LVMI against non-spironolactone therapy (− 9.5 g/m2 versus − 5.5 g/m2) in a retrospective propensity score-matched cohort study for hypertensive patients with HFpEF [48]. According to the meta-analyses assessing the effect on LVM of antihypertensive drug classes, ARBs, ACE inhibitors, and CCBs have the strongest LVM-regressive effect among ARBs, ACE inhibitors, CCBs, beta-blockers, and diuretics [8, 49]. Although MRBs were not compared in these meta-analyses, studies examining the effect of olmesartan in combination with azelnidipine or amlodipine in Japanese hypertensive patients with baseline LVMI of 123 g/m2 showed a reduction in LVMI ranging from − 3.0 to 6.5 g/m2 at 6 months [50]. Compared with the change of − 13.9 g/m2 (baseline 127.9 g/m2) in the RASi subcohort and − 8.2 g/m2 (baseline 113.9 g/m2) in the CCB subcohort in this study, it is possible that esaxerenone may also exert LVMI-lowering effects that are comparable to those of ARBs and CCBs. However, this will need to be clarified in future comparative studies.
The following mechanisms may underlie the marked reduction of LVMI by esaxerenone: (1) via BP reduction, (2) the pleiotropic effects of neurohormonal modulation, and (3) direct effects on myocardium. Although BP reduction is the primary mechanism, a previous report comparing the BP- and LVMI-lowering effects of esaxerenone before and after its administration suggested that the LVMI-lowering effect of esaxerenone might be also exerted by a mechanism independent of its antihypertensive effect [35]. Indeed, in the present study, the antihypertensive effect of esaxerenone reached steady state at approximately 6 weeks, whereas the LVMI-reducing effect persisted up to 24 weeks. This observation is consistent with the 4E-left ventricular hypertrophy study, in which the effect of eplerenone (alone or in combination with enalapril) on the change in BP and its effect on reducing LVM did not move in parallel [47]. Considering these results and the fact that the LVMI-lowering effect of esaxerenone was accompanied by a decrease in plasma BNP [35,36,37], we assume pleiotropic effects through neurohormonal modulation and/or direct effects on myocardium of esaxerenone. Recently, esaxerenone was found to reduce cardiac inflammation and oxidative stress in high salt-loaded rats, leading to improvement of cardiac remodeling and the reduction of fibrosis [33]. Future clinical and basic studies to elucidate the cardioprotective mechanisms of esaxerenone will provide deeper insights.
In this study, the baseline mean LVEF value of the patients was 62.8% within the normal range; however, LVEF tended to further increase with esaxerenone treatment and was significantly increased in the RASi subcohort. The previous reports of esaxerenone in hypertensive patients with HFpEF have shown a significant increase in LVEF at 6 months [35]. Moreover, esaxerenone prevented the deterioration of LVEF as well as worsening of fractional shortening and stroke volume in rats with salt-induced myocardial injury [33]. Thus, esaxerenone may have a favorable effect on left ventricular systolic function. Regarding the parameters recommended to assess left ventricular diastolic function [51], the baseline mean values except for LAVI were in the normal range (E/e′ of 9.7, TRV of 2.2 m/s, and LAVI of 41.9 ml/m2) [52]. LAVI tended to decrease in this study, but not significantly; E/A and E/e′ also tended to decrease, with significant decreases in E/A in the total population and RASi subcohort and in E/e′ in the total population and CCB subcohort; no change in TRV was observed. Esaxerenone has been reported to significantly reduce E/e′ in hypertensive patients with HFpEF [35], and a meta-analysis of steroidal MRBs also found significant reductions in E/A, E/e′, and LAVI for patients with HFpEF [53]. Esaxerenone might contribute to the prevention of left ventricular systolic and diastolic dysfunction and transition to HFpEF in patients with LVH.
Hypertensive patients with cardiac disease were not included in the Phase 3 studies of esaxerenone, and safety information was limited in this population. ESES-LVH was the first study to follow this patient population for 24 weeks, especially during the first 12 weeks, when serum potassium, eGFRcreat, and BP were monitored every 2 weeks. In the present study, the incidences of TEAEs and drug-related TEAEs were 35.0% and 3.3%, respectively, and were similar to those reported in a series of Phase 3 studies of esaxerenone including hypertensive patients with essential hypertension [22, 23], moderate renal impairment [25], type 2 diabetes with albuminuria [26, 28, 29], and primary aldosteronism [27]. In addition, new safety concerns were not identified compared with these previous studies. Regarding serum potassium elevation, a well-known adverse effect of MRBs [54], blood potassium increased occurred as a TEAE in two (3.3%) patients and was one of the most frequent TEAEs. All blood potassium increased events were drug-related TEAEs, one of which led to treatment discontinuation. The incidence of serum potassium level ≥ 5.5 mEq/l (5.0%) was similar to those reported in previous esaxerenone studies (3.0%−12.1% in hypertensive patients with essential hypertension, moderate renal dysfunction, and type 2 diabetes with albuminuria) [54]. Although treatment with a RASi is one of the risk factors for serum potassium elevation during esaxerenone administration [54], there was no difference in the time course change in serum potassium levels or the incidence of blood potassium increased between the RASi and CCB subcohorts. The time course change and the mean change in eGFRcreat were similar to the findings in previous studies of esaxerenone in hypertensive patients with various complications [22, 23, 25,26,27,28,29,30]. These results suggest that esaxerenone can be safely administered in hypertensive patients with various complications, including cardiac hypertrophy.
The present study has some limitations. First, because of the COVID-19 pandemic and torrential rain in the area of participating institutions, the sample size was small as approximately half of the initial target population could be enrolled. Therefore, the statistical power in the RASi and CCB subcohorts may have been insufficient. Second, this study had a one-arm design, lacking a placebo arm or an active comparator; as such, the reduction in BP and LVMI observed might be partly attributable to the placebo effect or factors beyond the drug treatment. However, although the LVMI-lowering effect remains to be addressed, previous controlled clinical trials of esaxerenone confirmed the significant antihypertensive action of the drug [28, 44]. Because this was an exploratory study, multiplicity was not adjusted, and no comparisons were made between the RASi and CCB subcohorts. The findings in this study may be used as a basis for future clinical studies with an active comparator design. In addition, multivariate analyses to identify factors affecting the efficacy and safety of esaxerenone are also warranted in the future. Third, as Japan is the only country that has approved esaxerenone as an antihypertensive agent to date, the present study on esaxerenone could only be conducted in Japan, and the generalizability of our findings to non-Asian populations should be interpreted with caution.
Conclusion
In hypertensive patients with cardiac hypertrophy who had an inadequate response to RASi or CCB, esaxerenone clearly demonstrated consistent BP-lowering and LVH-regressive effects regardless of the concomitant use of RASi or CCB. Furthermore, esaxerenone can be used safely in these patients without clinically relevant serum potassium elevation and eGFRcreat reduction. Therefore, esaxerenone may be a suitable antihypertensive treatment option accompanied by cardioprotective effects for hypertensive patients with LVH.
Data Availability
The anonymized data underlying the results presented in this manuscript may be made available to researchers upon submission of a reasonable request to the corresponding author. The decision to disclose the data will be made by the corresponding author and the funder, Daiichi Sankyo Co., Ltd. Data disclosure can be requested for 36 months from article publication.
References
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–616.
Muiesan ML, Salvetti M, Rizzoni D, Castellano M, Donato F, Agabiti-Rosei E. Association of change in left ventricular mass with prognosis during long-term antihypertensive treatment. J Hypertens. 1995;13:1091–5.
Pierdomenico SD, Cuccurullo F. Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens. 2010;23:876–81.
Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36:2284–309.
Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-248.
Soliman EZ, Ambrosius WT, Cushman WC, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (Systolic Blood Pressure Intervention Trial). Circulation. 2017;136:440–50.
Miller AB, Reichek N, St John SM, et al. Importance of blood pressure control in left ventricular mass regression. J Am Soc Hypertens. 2010;4:302–10.
Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115:41–6.
Umemura S, Arima H, Arima S, et al. The Japanese Society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering treatment. 6. Prevention of heart failure and new-onset heart failure–meta-analyses of randomized trials. J Hypertens. 2016;34:373–84.
Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65:257–63.
Sueta D, Yamamoto E, Tsujita K. Mineralocorticoid receptor blockers: Novel selective nonsteroidal mineralocorticoid receptor antagonists. Curr Hypertens Rep. 2020;22:21.
Tezuka Y, Ito S. The time to reconsider mineralocorticoid receptor blocking strategy: arrival of nonsteroidal mineralocorticoid receptor blockers. Curr Hypertens Rep. 2022;24:215–24.
Catena C, Colussi G, Brosolo G, Iogna-Prat L, Sechi LA. Aldosterone and aldosterone antagonists in cardiac disease: what is known, what is new. Am J Cardiovasc Dis. 2012;2:50–7.
Ferreira JP, Rossello X, Eschalier R, et al. MRAs in elderly HF patients: Individual patient-data meta-analysis of RALES, EMPHASIS-HF, and TOPCAT. JACC Heart Fail. 2019;7:1012–21.
Maron MS, Chan RH, Kapur NK, et al. Effect of spironolactone on myocardial fibrosis and other clinical variables in patients with hypertrophic cardiomyopathy. Am J Med. 2018;131:837–41.
Krasińska B, Cofta S, Szczepaniak-Chicheł L, et al. The effects of eplerenone on the circadian blood pressure pattern and left ventricular hypertrophy in patients with obstructive sleep apnea and resistant hypertension-a randomized, controlled trial. J Clin Med. 2019;8:1671.
Trinchieri A, Perletti G, Magri V, Stamatiou K, Trinchieri M, Montanari E. Drug-induced gynecomastia: a systematic review and meta-analysis of randomized clinical trials. Arch Ital Urol Androl. 2021;93:489–96.
Wan N, Rahman A, Nishiyama A. Esaxerenone, a novel nonsteroidal mineralocorticoid receptor blocker (MRB) in hypertension and chronic kidney disease. J Hum Hypertens. 2021;35:148–56.
Viatris Pharmaceuticals Japan Inc. SELARA (eplerenone) Tablets, package insert. 2023. https://www.info.pmda.go.jp/go/pack/2149045F1029_4_04/ (In Japanese)
Pfizer Inc. INSPRA (eplerenone) tablets, package insert. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021437s013lbl.pdf
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study). Hypertension. 2020;75:51–8.
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42:1932–41.
Rakugi H, Ito S, Ito H, Okuda Y, Iijima S. The efficacy and safety of esaxerenone for patients with grade III hypertension. Prog Med. 2020;40:755–60 (In Japanese).
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44:489–97.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42:1572–81.
Satoh F, Ito S, Itoh H, et al. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res. 2021;44:464–72.
Ito S, Kashihara N, Shikata K, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15:1715–27.
Ito S, Kashihara N, Shikata K, et al. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study. Clin Exp Nephrol. 2021;25:1070–8.
Uchida HA, Nakajima H, Hashimoto M, et al. Efficacy and safety of esaxerenone in hypertensive patients with diabetic kidney disease: a multicenter, open-label, prospective study. Adv Ther. 2022;39:5158–75.
Shikata K, Ito S, Kashihara N, et al. Reduction in the magnitude of serum potassium elevation in combination therapy with esaxerenone (CS-3150) and sodium-glucose cotransporter 2 inhibitor in patients with diabetic kidney disease: subanalysis of two phase III studies. J Diabetes Investig. 2022;13:1190–202.
Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42:293–300.
Rahman A, Sawano T, Sen A, et al. Cardioprotective effects of a nonsteroidal mineralocorticoid receptor blocker, esaxerenone, in Dahl salt-sensitive hypertensive rats. Int J Mol Sci. 2021;22:2069.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73.
Imamura T, Oshima A, Narang N, Kinugawa K. Implication of mineralocorticoid receptor antagonist esaxerenone in patients with heart failure with preserved ejection fraction. Circ Rep. 2021;3:660–5.
Naruke T, Maemura K, Oki T, et al. Efficacy and safety of esaxerenone in patients with hypertension and concomitant heart failure. Hypertens Res. 2021;44:601–3.
Iwahana T, Saito Y, Okada S, Kato H, Ono R, Kobayashi Y. Safety and efficacy of esaxerenone in Japanese hypertensive patients with heart failure with reduced ejection fraction: a retrospective study. PLoS ONE. 2021;16: e0259485.
Sueta D, Yamamoto E, Usuku H, et al. Rationale and design of the efficacy and safety of esaxerenone in hypertensive patients with left Ventricular hypertrophy (ESES-LVH) Study – protocol for a multicenter, open-label, exploratory interventional study. Circ Rep. 2022;4:99–104.
Daiichi Sankyo Co., Ltd. MINNEBRO (esaxerenone) OD tablets, package insert. 2023 https://www.info.pmda.go.jp/go/pack/2149049F1027_1_08/. (In Japanese)
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8.
Kario K, Saito I, Kushiro T, et al. Effect of the angiotensin II receptor antagonist lmesartan on morning home blood pressure in hypertension: HONEST study at 16 weeks. J Hum Hypertens. 2013;27:721–8.
Toh N, Ishii K, Kihara H, et al. Effect of diuretic or calcium-channel blocker plus agiotensin-receptor blocker on diastolic function in hypertensive patients. Circ J. 2016;80:426–34.
Ito S, Itoh H, Rakugi H, Okuda Y, Yamakawa S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens. 2019;33:542–51.
Kario K, Nishizawa M, Kato M, et al. Nighttime home blood pressure lowering effect of esaxerenone in patients with uncontrolled nocturnal hypertension: the EARLY-NH study. Hypertens Res. 2023;46:1782–94.
Kobayashi Y, Haze T, Yano Y, et al. JPAS/JRAS Study Group. Associations between changes in plasma renin activity and aldosterone concentrations and changes in kidney function after treatment for primary aldosteronism. Kidney Int Rep. 2020;5:1291–7.
Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–8.
Gu J, Fan YQ, Han ZH, et al. Association between long-term prescription of aldosterone antagonist and the progression of heart failure with preserved ejection fraction in hypertensive patients. Int J Cardiol. 2016;220:56–60.
Salvetti M, Paini A, Bertacchini F, et al. Changes in left ventricular geometry during antihypertensive treatment. Pharmacol Res. 2018;134:193–9.
Takami T, Saito Y. Azelnidipine plus olmesartan versus amlodipine plus olmesartan on arterial stiffness and cardiac function in hypertensive patients: a randomized trial. Drug Des Devel Ther. 2013;7:175–83.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314.
Kapelios CJ, Murrow JR, Nührenberg TG, Montoro Lopez MN. Effect of mineralocorticoid receptor antagonists on cardiac function in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:367–77.
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res. 2021;44:371–85.
Acknowledgements
We thank the participants of this study.
Medical Writing/Editorial Assistance.
We thank Michelle Belanger, MD, of Edanz (www.edanz.com), for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd., in accordance with Good Publication Practice 2022 guidelines (https://www.ismpp.org/gpp-2022).
Funding
The ESES-LVH study was supported by Daiichi Sankyo Co., Ltd., Tokyo, Japan. Daiichi Sankyo Co., Ltd. was involved in the study design, planning of the data analysis, data interpretation, and development of the manuscript, but was not involved in the data management and statistical analysis. CMIC Co., Ltd. was directly involved in the data management and statistical analysis. The journal’s Rapid Service and Open Access Fees were funded by the study sponsor, Daiichi Sankyo Co., Ltd.
Author information
Authors and Affiliations
Consortia
Contributions
Eiichiro Yamamoto, Hiroki Usuku, Daisuke Sueta, Satoru Suzuki, Taishi Nakamura, Kunihiko Matsui, Kenichi Matsushita, and Kenichi Tsujita contributed to the study design and planning of data analysis, conduct of the study, data interpretation, and writing/reviewing the manuscript. Tomoko Iwasaki, Naritsugu Sakaino, Toshihiko Sakanashi, Kazuto Hirayama, Hirofumi Kurokawa, Koichi Kikuta, Nobuyasu Yamamoto, Koji Sato, and Takanori Tokitsu contributed to the conduct of the study and writing/reviewing the manuscript. Takashi Taguchi and Kotaro Sugimoto contributed to the study design and planning of data analysis, data interpretation, and writing/reviewing the manuscript. Kazuhito Shiosakai contributed to the study design and planning of data analysis and writing/reviewing the manuscript. All authors gave their final approval of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Eiichiro Yamamoto, Hiroki Usuku, Daisuke Sueta, Satoru Suzuki, Taishi Nakamura, Kunihiko Matsui, Kenichi Matsushita, Tomoko Iwasaki, Naritsugu Sakaino, Toshihiko Sakanashi, Kazuto Hirayama, Hirofumi Kurokawa, Koichi Kikuta, Nobuyasu Yamamoto, and Takanori Tokitsu have no conflicts of interest to disclose. Koji Sato has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational event fees from Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd. Takashi Taguchi, Kazuhito Shiosakai, and Kotaro Sugimoto are employees of Daiichi Sankyo Co., Ltd. Kenichi Tsujita has received grant fees from PPD-Shin Nippon Biomedical Laboratories K.K., Alexion Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Abbott Medical Japan LLC., Bayer Yakuhin, Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., and ITI Co., Ltd.; payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational event fees from Abbott Medical Japan LLC., Amgen K.K., AstraZeneca K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., Medtronic Japan Co., Ltd., Kowa Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Janssen Pharmaceutical K.K.; and has other financial or non-financial interests with Abbott Japan LLC., Boston Scientific Japan K.K., Fides-one Inc., GM Medical Co., Ltd., ITI Co., Ltd., Kaneka Medix Corp., Nipro Corp., Terumo Corp., Abbott Medical Japan LLC., Boston Scientific Japan K.K., Cardinal Health K.K., Fides-one Inc., FUKUDA DENSHI Co., Ltd., Japan Lifeline Co., Ltd., Medical Appliance Co., Ltd., and Medtronic Japan Co., Ltd.
Ethical Approval
The protocol for this study was approved by the Kumamoto University Certified Clinical Research Review Board (CRB7200002) and prospectively registered with the Japan Registry of Clinical Trials (jRCTs071190043; https://jrct.niph.go.jp/en-latest-detail/jRCTs071190043). The study was conducted in accordance with the Declaration of Helsinki and the Clinical Trials Act in Japan. All the study participants provided written informed consent before enrollment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yamamoto, E., Usuku, H., Sueta, D. et al. Efficacy and Safety of Esaxerenone in Hypertensive Patients with Left Ventricular Hypertrophy (ESES-LVH) Study: A Multicenter, Open-Label, Prospective, Interventional Study. Adv Ther 41, 1284–1303 (2024). https://doi.org/10.1007/s12325-024-02780-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02780-6