Abstract
Introduction
Teclistamab is the first approved B cell maturation antigen × CD3 bispecific antibody with precision dosing for the treatment of triple-class exposed (TCE) relapsed/refractory multiple myeloma (RRMM). We compared the effectiveness of teclistamab in MajesTEC-1 versus real-world physician’s choice of therapy (RWPC) in patients from the prospective, non-interventional LocoMMotion and MoMMent studies.
Methods
Patients treated with teclistamab from MajesTEC-1 (N = 165) were compared with an external control arm from LocoMMotion (N = 248) or LocoMMotion + MoMMent pooled (N = 302). Inverse probability of treatment weighting adjusted for imbalances in prognostic baseline characteristics. The relative effect of teclistamab versus RWPC for overall response rate (ORR), very good partial response or better (≥ VGPR) rate, and complete response or better (≥ CR) rate was estimated with an odds ratio using weighted logistic regression transformed into a response-rate ratio (RR) and 95% confidence interval (CI). Weighted proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs for duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
Results
Baseline characteristics were well balanced between treatment cohorts after reweighting. Patients treated with teclistamab had significantly improved outcomes versus RWPC in LocoMMotion: ORR (RR [95% CI], 2.44 [1.79–3.33]; p < 0.0001), ≥ VGPR (RR 5.78 [3.74–8.93]; p < 0.0001), ≥ CR (RR 113.73 [15.68–825.13]; p < 0.0001), DOR (HR 0.39 [0.24–0.64]; p = 0.0002), PFS (HR 0.48 [0.35–0.64]; p < 0.0001), and OS (HR 0.64 [0.46–0.88]; p = 0.0055). Teclistamab versus RWPC in LocoMMotion + MoMMent also had significantly improved outcomes: ORR (RR 2.41 [1.80–3.23]; p < 0.0001), ≥ VGPR (RR 5.91 [3.93–8.88]; p < 0.0001), ≥ CR (RR 132.32 [19.06–918.47]; p < 0.0001), DOR (HR 0.43 [0.26–0.71]; p = 0.0011), PFS (HR 0.49 [0.37–0.66]; p < 0.0001), and OS (HR 0.69 [0.50–0.95]; p = 0.0247).
Conclusion
Teclistamab demonstrated significantly improved effectiveness over RWPC in LocoMMotion ± MoMMent, emphasizing its clinical benefit as a highly effective treatment for patients with TCE RRMM.
Trial Registration
MajesTEC-1, ClinicalTrials.gov NCT03145181 (phase 1) and NCT04557098 (phase 2); LocoMMotion, ClinicalTrials.gov NCT04035226; MoMMent, ClinicalTrials.gov NCT05160584.
Similar content being viewed by others
Why carry out this study? |
Previously, a comparative analysis between the B cell maturation antigen × CD3 bispecific antibody teclistamab in the ongoing phase 1/2 MajesTEC-1 study and real-world physician’s choice of therapy (RWPC) in the prospective, non-interventional LocoMMotion study demonstrated improved effectiveness with teclistamab versus RWPC for patients with triple-class exposed (TCE) relapsed/refractory multiple myeloma (RRMM). |
The prospective, non-interventional MoMMent study was initiated as a complement to LocoMMotion to enable data pooling and reflect the most recent treatments used in clinical practice. |
The objectives of this study were to assess the comparative effectiveness of teclistamab in the MajesTEC-1 study versus RWPC in patients with TCE RRMM from the LocoMMotion study alone using extended follow-up data from both studies, and to assess comparative effectiveness of teclistamab versus RWPC from the pooled LocoMMotion + MoMMent dataset for the first time. |
What was learned from the study? |
Patients treated with teclistamab in MajesTEC-1 had significant improvements in all evaluated efficacy outcomes (overall response rate, complete response or better rate, very good partial response or better rate, duration of response, progression-free survival, overall survival) compared with eligibility-matched patient cohorts treated with RWPC from both the LocoMMotion study alone and the LocoMMotion + MoMMent pooled dataset. |
The results indicate that teclistamab demonstrated significantly improved effectiveness over RWPC in LocoMMotion alone and pooled LocoMMotion + MoMMent, emphasizing its clinical benefit as a highly effective treatment for patients with TCE RRMM, who have had historically limited therapeutic options and poor outcomes. |
Introduction
Advances in treatment options that have occurred in recent decades, including the use of immunomodulatory agents (IMiDs), proteasome inhibitors (PIs), and monoclonal antibodies (mAbs), have significantly improved outcomes in patients with multiple myeloma (MM) [1,2,3]. Despite the use of these widely available treatments, patients with MM eventually relapse or become refractory and require further lines of therapy (LOTs) [4, 5]. With each additional LOT, efficacy outcomes worsen, and toxicities and comorbidities become more prevalent [6]. Patients who have previously received treatment with a PI, an IMiD, and an anti-CD38 mAb (i.e., are triple-class exposed [TCE]) have an especially poor prognosis with low likelihood of response to subsequent therapy and shortened time to progression or death [4, 5, 7]. Previous real-world studies in this difficult-to-treat patient population have shown overall response rates (ORRs) of approximately 30% and median progression-free survival (PFS) of just 3–5 months with treatments used in real-world clinical practice [7, 8]. Therefore, effective therapies with novel mechanisms of action are needed to improve outcomes and prevent relapse in patients with TCE relapsed/refractory MM (RRMM).
The rapid evolution of the MM therapeutic landscape is evidenced by the emergence of several new, highly effective treatment classes, including chimeric antigen receptor (CAR)-T cells [9,10,11,12,13,14], bispecific antibodies [15,16,17,18,19,20,21,22], and selective inhibitors of nuclear export [23,24,25] that have gained regulatory approval in recent years. Teclistamab is the first approved B cell maturation antigen (BCMA) × CD3 bispecific antibody with precision dosing for the treatment of patients with TCE RRMM [18, 19]. In the pivotal phase 1/2 MajesTEC-1 study (NCT03145181/NCT04557098), teclistamab demonstrated rapid, deep, and durable responses in patients with TCE RRMM [15]. At a median follow-up of 22.8 months, the ORR was 63%, 45.5% of patients achieved a complete response or better (≥ CR), and median duration of response (DOR) was 21.6 months [26]. As MajesTEC-1 is a single-arm study, adjusted treatment comparisons can be used to assess the effectiveness of teclistamab relative to real-world physician’s choice of therapy (RWPC) [27, 28].
LocoMMotion is the first prospective, multinational, non-interventional study of RWPC in TCE RRMM [7]. A previous comparative analysis between MajesTEC-1 and LocoMMotion demonstrated significantly improved effectiveness with teclistamab over RWPC [27]. As the RRMM treatment landscape is quickly evolving, continual assessments of the patient population, available treatments, and outcomes are necessary to fully address unmet needs in clinical practice [1]. The prospective, multinational, non-interventional MoMMent study (NCT05160584) was initiated as a complementary continuation of LocoMMotion to capture the most recently approved therapies used in clinical practice in the same patient population and to enable pooling with LocoMMotion data. To our knowledge, the pooled LocoMMotion + MoMMent dataset reflects the most updated evidence of RWPC in TCE RRMM over the past 3 years. Both LocoMMotion and MoMMent were specifically designed as external control arms mirroring several ongoing single-arm trials, including MajesTEC-1, to serve as the benchmark for comparison with novel therapies [29, 30]. Here, we report the comparative effectiveness of teclistamab versus RWPC in patients with TCE RRMM from the LocoMMotion study alone, with extended follow-up from MajesTEC-1 and LocoMMotion, and from the pooled LocoMMotion + MoMMent studies for the first time.
Methods
Patient Populations
Individual patient data (IPD) from MajesTEC-1 (NCT03145181, NCT04557098), LocoMMotion (NCT04035226), and MoMMent (NCT05160584) were used to conduct adjusted comparisons between teclistamab and RWPC (Table 1). IPD from MajesTEC-1 included all patients treated with teclistamab subcutaneous 1.5 mg/kg weekly (N = 165), including those who switched to a less frequent (every other week or monthly) dosing schedule, and were compared with data from patients in LocoMMotion alone (N = 248; enrolled August 2019–October 2020) or LocoMMotion + MoMMent pooled (N = 302, MoMMent enrolled November 2021–July 2022). The index date for all studies was the date of treatment initiation.
All patients included in the comparative effectiveness analyses were aligned with key inclusion and exclusion criteria from the MajesTEC-1 study. Eligible patients had measurable disease as defined by International Myeloma Working Group consensus criteria [31]; received prior treatment with an IMiD, a PI, and an anti-CD38 mAb (i.e., were TCE); progressive disease within ≤ 12 months of their last LOT; and Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. The same comparative analyses were conducted applying additional eligibility criteria from MajesTEC-1 to the comparator cohorts (i.e., receiving ≥ 3 prior LOT, no prior exposure to any BCMA-targeted therapy nor bispecifics, hemoglobin ≥ 8 g/dL, and creatinine clearance ≥ 40 mL/min/1.73 m2), reducing the RWPC cohorts LocoMMotion and LocoMMotion + MoMMent to 136 and 170 patients, respectively.
For all studies, patients provided written informed consent, and an independent ethics committee or institutional review board at each study center approved the study protocol (Supplementary Material Tables 1, 2, 3). MajesTEC-1 was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. The LocoMMotion and MoMMent studies were conducted in accordance with the Declaration of Helsinki.
Efficacy Assessment
Responses were evaluated by an independent review committee in MajesTEC-1 and a response review committee in LocoMMotion and MoMMent. ORR, rates of very good partial response or better (≥ VGPR) and ≥ CR, DOR, PFS, and overall survival (OS) were compared between teclistamab and RWPC.
Statistical Analyses
In the primary analysis, inverse probability of treatment weighting (IPTW), using the average treatment effect in the treated (ATT) approach, was implemented to adjust for imbalances in baseline covariates of prognostic significance between the teclistamab and RWPC cohorts (base case) [32]. Prognostic baseline characteristics for adjustment in the base case analyses were selected a priori on the basis of literature review and consultations with clinical experts, and included refractory status, International Staging System (ISS) stage, time to progression on previous LOT, presence of extramedullary disease (EMD), number of prior LOTs, time since diagnosis, average duration of previous LOTs, age, hemoglobin levels, lactate dehydrogenase levels, creatinine clearance, ECOG performance status, sex, MM type, and previous hematopoietic stem cell transplant status.
Cytogenetic risk was not included in the primary adjusted analyses because of a high rate of missingness in both LocoMMotion and LocoMMotion + MoMMent pooled (37.2% and 44.9%, respectively). Race was also not included in the adjusted analyses as a result of high weights assigned to the small number of patients of color enrolled in LocoMMotion to account for the higher proportion of patients of color in MajesTEC-1, which induced unstable estimates and increased imbalance for other factors. Sensitivity analyses were run, including race and type of cytogenetic profile in the ATT adjustment, in addition to the variables included in the base case.
The ATT approach involved two steps. First, propensity scores estimated using multivariable logistic regression, which included the prognostic baseline characteristics as adjustment variables, were transformed into ATT weights assigned to the RWPC cohorts to balance baseline factors across sites. The degree of imbalance between the groups was assessed using standardized mean differences (SMDs), with values > 0.2 considered to reflect important differences. In a second step, weighted logistic regression was used to estimate odds ratios (ORs), response-rate ratios (RRs), and corresponding 95% confidence intervals (CIs) [33] to evaluate the relative effect of teclistamab versus RWPC for ORR and rates of ≥ VGPR and ≥ CR. Weighted Cox proportional hazards regression was used to estimate hazard ratios (HRs) with corresponding 95% CIs for DOR, PFS, and OS. If violations of the proportional hazards assumption were encountered, time-dependent HRs would be estimated.
Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and R versions 3.6.1 and 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
The median follow-up was 22.8 months in MajesTEC-1 (data cutoff January 4, 2023), 26.4 months in LocoMMotion (data cutoff October 27, 2020), and 24.3 months in LocoMMotion + MoMMent pooled (data cutoff March 13, 2023), including a median follow-up of 9.3 months in MoMMent alone. Before reweighting, differences were observed in several base case variables, with the teclistamab cohort having a higher proportion of patients who were < 65 years of age (52.1% vs 35.5%), were penta-drug refractory (30.3% vs 16.9%), had ISS stage I disease (52.7% vs 32.3%), had EMD (17.0% vs 9.7%), had a creatinine clearance of 60 to < 90 mL/min (44.2% vs 35.1%) or ≥ 90 mL/min (29.1% vs 25.0%), immunoglobulin G (IgG) subtype (55.2% vs 40.3%), and had previous hematopoietic stem cell transplantation (81.8% vs 64.5%) compared with the LocoMMotion RWPC cohort (Table 2).
Differences in base case variables before reweighting were also observed between the MajesTEC-1 and LocoMMotion + MoMMent pooled cohorts, with a higher proportion of patients treated with teclistamab who were penta-drug refractory (30.3% vs 17.5%), < 65 years of age (52.1% vs 34.1%), ISS stage I disease (52.7% vs 31.8%), had EMD (17.0% vs 9.9%), had a creatinine clearance of 60 to < 90 mL/min (44.2% vs 36.4%) or ≥ 90 mL/min (29.1% vs 23.5%), ECOG performance status of 0 (33.3% vs 23.5%), IgG subtype (55.2% vs 40.7%), and had previous hematopoietic stem cell transplantation (81.8% vs 63.9%) compared with RWPC.
After reweighting, baseline characteristics were well balanced between the teclistamab cohort and the RWPC cohorts in both LocoMMotion and pooled LocoMMotion + MoMMent, with most SMD values < 0.10 and a maximum value of 0.16 for both RWPC datasets in the base case.
Treatment Regimens Received in Real-World Clinical Practice
A total of 91 and 102 unique treatment regimens were used in the LocoMMotion study alone [34] and the LocoMMotion + MoMMent pooled dataset, respectively. The most common treatments included combinations of PIs, IMiDs, mAbs, alkylating agents, and corticosteroids, with the most common regimen being pomalidomide–cyclophosphamide–dexamethasone (Table 3). Only 4 (1.6%) patients received belantamab mafodotin as index therapy in LocoMMotion, which increased to 15 (5.0%) with the addition of patients from MoMMent, as it had become available during the LocoMMotion enrollment period. Similarly, only four patients received CAR-T cell therapy (idecabtagene vicleucel), all of whom were from the MoMMent study, as it was not approved until after the end of LocoMMotion enrollment.
At the time of data cutoff, 152 patients from the LocoMMotion study had received subsequent anti-myeloma therapy. Of these, 76 (50%) received ≥ 1 novel agent, most commonly belantamab mafodotin (n = 49) and selinexor (n = 21). Some patients received bispecific antibodies (n = 14) or CAR-T cell therapy (n = 3) as subsequent therapy, both of which were part of a clinical trial. With the addition of MoMMent, a total 185 patients had received subsequent anti-myeloma therapy at the time of data cutoff in the pooled dataset, of whom 94 (51%) received ≥ 1 novel agent. The most common novel agents received as subsequent therapy remained belantamab mafodotin (n = 53) and selinexor (n = 21), with more patients receiving bispecific antibodies (n = 24) and CAR-T cell therapies (n = 6).
Comparative Analysis of Efficacy Outcomes
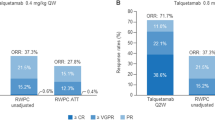
The observed ORR for teclistamab was 63.0% versus 31.9% with RWPC in LocoMMotion and 31.8% with RWPC in the LocoMMotion + MoMMent pooled dataset (Fig. 1). Additionally, responders on teclistamab reached deeper levels of response than LocoMMotion RWPC responders. After IPTW-ATT adjustment, ORR (63.0% vs 25.9%; RR 2.44 [95% CI] 1.79–3.33, p < 0.0001), ≥ VGPR (59.4% vs 10.3%; RR 5.78 [3.74–8.93], p < 0.0001), and ≥ CR (45.5% vs 0.4%; RR 113.73 [15.68–825.13], p < 0.0001) were significantly higher with teclistamab versus LocoMMotion RWPC.
Unadjusted and ATT-adjusted response rates for teclistamab versus LocoMMotion versus pooled LocoMMotion + MoMMent. aORR = ≥ CR + VGPR + PR; may not sum appropriately as shown because of rounding. bTeclistamab versus LocoMMotion. cTeclistamab vs LocoMMotion + MoMMent. ATT average treatment effect in the treated, CI confidence interval, ≥ CR complete response or better, ORR overall response rate, PR partial response, RR response-rate ratio, RWPC real-world physician’s choice of therapy, ≥ VGPR very good partial response or better
Similar results were observed with comparisons to the pooled LocoMMotion + MoMMent dataset. After IPTW-ATT adjustment, ORR (63.0% vs 26.2%; RR 2.41 [95% CI] 1.80–3.23, p < 0.0001), ≥ VGPR (59.4% vs 10.1%; RR 5.91 [3.93–8.88], p < 0.0001), and ≥ CR (45.5% vs 0.3%; RR 132.32 [19.06–918.47], p < 0.0001) were significantly higher with teclistamab versus RWPC from LocoMMotion + MoMMent pooled. Results of the sensitivity analysis were consistent with those of the base case (Table 4).
ATT-adjusted DOR (median 21.55 months vs 7.29 months; HR 0.39 [0.24–0.64], p = 0.0002), PFS (median 11.30 months vs 4.07 months; HR 0.48 [0.35–0.64], p < 0.0001), and OS (median 21.91 months vs 11.76 months; HR 0.64 [0.46–0.88], p = 0.0055) were significantly longer with teclistamab versus LocoMMotion RWPC (Fig. 2, Supplementary Material Fig. 1). Similarly, ATT-adjusted DOR (median 21.55 months vs 8.11 months; HR 0.43 [0.26–0.71], p = 0.0011), PFS (median 11.30 months vs 4.07 months; HR 0.49 [0.37–0.66], p < 0.0001), and OS (median 21.91 months vs 13.27 months; HR 0.69 [0.50–0.95], p = 0.0247) were significantly longer with teclistamab versus pooled LocoMMotion + MoMMent RWPC. Results of the sensitivity analysis were consistent with those of the base case (Table 5). Proportional hazards assumption was met for all endpoints, and time-dependent HRs were therefore not estimated. Comparative results when applying additional eligibility criteria to the RWPC cohorts were consistent with the main results across all endpoints (Supplementary Material Tables 4, 5).
Base case adjusted (ATT weighted) Kaplan–Meier plots for a DOR, b PFS, and c OS for teclistamab versus LocoMMotion versus pooled LocoMMotion + MoMMent. ATT average treatment effect in the treated, CI confidence interval, DOR duration of response, NE not evaluable, OS overall survival, PFS progression-free survival
Discussion
The therapeutic landscape of MM is rapidly evolving, and treatment advances in recent decades have given rise to the introduction of PIs, IMiDs, and anti-CD38 mAbs, which now form the foundation of current RWPC [1,2,3,4]. Patients with RRMM who have received all three of these drug classes (i.e., are TCE) have already exhausted most of their therapeutic options and tend to have poor outcomes [5, 7]. A high unmet medical need remains for therapies with novel mechanisms of action that produce deeper and more durable responses with improved survival outcomes for this difficult-to-treat population for whom there are currently limited options [7, 8]. Teclistamab, the first approved BCMA × CD3 bispecific antibody for patients with TCE RRMM, has demonstrated robust efficacy in MajesTEC-1 [15, 18, 19, 26]. Because MajesTEC-1 is a single-arm study, and there are currently no data available from randomized controlled clinical trials of teclistamab, adjusted comparisons can be used to identify the most effective treatment options to improve patient outcomes by controlling for differences in prognostic baseline characteristics between the populations being compared [35, 36]. The adjusted comparisons presented here evaluate the comparative effectiveness of teclistamab versus RWPC in the prospective, non-interventional, multinational LocoMMotion and MoMMent studies.
Patients in LocoMMotion were enrolled from August 2019 through October 2020, while those in the complementary MoMMent study were enrolled from November 2021 through July 2022. With the addition of MoMMent, the pooled RWPC cohort includes more recently available treatments, reflecting the evolution of treatments used in clinical practice over the past 3 years. LocoMMotion and MoMMent were specifically designed as external control arms mimicking the study designs and capturing a wide range of clinically relevant prognostic baseline factors and endpoints of several ongoing single-arm trials including MajesTEC-1 [13, 15, 16], allowing for robust comparative analyses. The pooled dataset therefore represents the most up-to-date RWPC currently available in TCE RRMM, serving as a benchmark for comparisons with novel therapies, for which no data from randomized controlled trials are yet available. While MoMMent data encompass more advanced RWPC therapies including novel immunotherapies like antibody–drug conjugates and monoclonal antibodies, there is still limited uptake of the most novel treatments, such as bispecific antibodies and CAR-T cell therapies.
A previously published adjusted comparison demonstrated significantly improved response and survival outcomes with teclistamab over RWPC in LocoMMotion [27]. With approximately 9 to 10 months of additional median follow-up reported here for both MajesTEC-1 (22.8 months total) and LocoMMotion (26.4 months total), our analysis of teclistamab versus RWPC in LocoMMotion confirms and expands upon those findings. Patients who received teclistamab in MajesTEC-1 were 2.4-fold, approximately 6-fold, and > 100-fold more likely to respond, achieve ≥ VGPR, and achieve ≥ CR, respectively, than those who received RWPC in LocoMMotion alone and LocoMMotion + MoMMent pooled. The improved depth of response observed with teclistamab is clear, as almost all the responders in MajesTEC-1 achieved ≥ VGPR (98/104), with the majority reaching ≥ CR (75/104). In contrast, less than half of those who responded to RWPC achieved ≥ VGPR in the LocoMMotion-only and LocoMMotion + MoMMent pooled datasets, both with and without ATT adjustment. Only a single patient in LocoMMotion (and none in MoMMent) reached CR. This observation may also be related to the lack of bone marrow-based evaluations to confirm CR outside of clinical trials. Responses with teclistamab were also significantly more durable: median DOR was approximately three times longer (21.55 months) versus RWPC in LocoMMotion (7.29 months) or LocoMMotion + MoMMent (8.11 months), with a 61% or 57% reduction, respectively, in the risk of progression or death compared with responders in the RWPC cohorts. The treatment benefit of teclistamab is further underscored by statistically significant improvements in all evaluated time-to-event outcomes. The median PFS with teclistamab is nearly three times longer (11.30 months) than with RWPC in both the LocoMMotion-only and pooled datasets (4.07 months). The median OS with teclistamab (21.91 months) is nearly double that with RWPC in LocoMMotion (11.76 months) or LocoMMotion + MoMMent pooled (13.27 months). These results demonstrate that, within the rapidly evolving MM treatment landscape, teclistamab still offers a significantly more effective treatment option than currently available RWPC. It is important to acknowledge, however, that only treatments that were approved during the enrollment period are included here as RWPC, which may not include more recently approved therapeutic options now available to patients.
Some limitations of our study must be considered. This analysis focused on comparisons between MajesTEC-1 and LocoMMotion alone and between MajesTEC-1 and LocoMMotion + MoMMent pooled; comparisons between MajesTEC-1 and MoMMent alone were outside the scope of these analyses. Another limitation of these analyses is that residual confounding cannot be excluded, as is the case with any non-randomized study. As both LocoMMotion and MoMMent were prospective studies, all clinically relevant patient characteristics available in the MajesTEC-1 study were captured, allowing for appropriate adjustment of prognostic factors. These factors were a priori identified from literature review and consultation with clinical experts and further validated by analyses of prognostic strength in the MajesTEC-1 and RWPC cohorts. Imbalances between cohorts for these factors were minimal after ATT adjustment, emphasizing the robustness of the analyses. An additional limitation is the omission of cytogenetic risk (due to high missingness) and race (due to low numbers of patients of color in the RWPC cohorts) from the primary analyses; however, the addition of cytogenetic risk and race as covariates in the sensitivity analyses did not affect the comparisons, and results were consistent with the base case. Notably, the high missingness of cytogenetic risk suggests that it is not systematically assessed in clinical practice. Additionally, results were nearly identical when further applying more detailed inclusion criteria from MajesTEC-1.
Another limitation of this study was the absence of a clear standard of care used in LocoMMotion and MoMMent. While the large number of treatments precluded comparisons of teclistamab versus individual therapies, the comparator groups were representative of regimens that were available to physicians for use in clinical practice at the time of the analysis. The large number of therapies in the RWPC cohorts also precluded comparisons of the safety profiles between individual treatments. The most common adverse events for teclistamab in MajesTEC-1 included cytopenias and infections, both of which are characteristic of MM and other anti-myeloma therapies; cytokine release syndrome (CRS) was also common following teclistamab treatment in MajesTEC-1, which is a class effect of T cell engaging bispecific antibodies as well as CAR-T cell therapies [7, 15, 37].
A key strength is the addition of the MoMMent study, including newer therapies that have recently been approved for patients with TCE RRMM. The selective inhibitor of nuclear export selinexor is only widely available in the USA [23, 24, 38] while the majority of patients in LocoMMotion and MoMMent were enrolled from Europe. The BCMA-targeting antibody–drug conjugate belantamab mafodotin was approved just 3 months before the end of the LocoMMotion enrollment period [39]; marketing authorization in the USA has since been withdrawn [40], and the Committee for Medicinal Products for Human Use has recently recommended to not renew its conditional marketing authorization in Europe [41]. The CAR-T cell therapies idecabtagene vicleucel [9, 10] and ciltacabtagene autoleucel [11, 12] were only approved during the MoMMent enrollment period; however, only 4 patients received CAR-T cell therapy in the MoMMent study. Since the end of the MoMMent enrollment period, 2 new treatments have received regulatory approval and are therefore not included in this analysis. The BCMA-directed bispecific antibody elranatamab [20] was approved in the USA in August 2023, and talquetamab became the first bispecific antibody targeting G protein-coupled receptor family C group D to be approved in the USA [21] and Europe [22] in August 2023. Some of these novel agents, including CAR-T cell therapy and bispecific antibodies, were received as part of subsequent therapy, reflected in the improvements in OS observed over time with the addition of MoMMent. Most of these novel therapies are still only widely accessible through participation in clinical trials, and additional real-world evidence is needed to continue to evaluate and optimize the use of available therapies for patients with RRMM. Teclistamab is an off-the-shelf, readily available treatment option for patients who may not be eligible for or who have limited access to cellular therapies [15, 42]. As the first approved bispecific antibody for TCE RRMM, teclistamab has a growing body of real-world clinical experiences to further inform treatment of this new class of therapy.
Conclusion
LocoMMotion and MoMMent, two prospective studies designed specifically to serve as benchmarks for comparison with novel therapies such as teclistamab, represent high-quality evidence reflecting RWPC in TCE (PI, IMiD, and anti-CD38 mAb) RRMM between 2019 and 2022. The adjusted treatment comparisons reported here demonstrated that teclistamab significantly improved effectiveness over RWPC in LocoMMotion alone and pooled LocoMMotion + MoMMent, emphasizing its clinical benefit as a highly effective treatment for patients with TCE RRMM.
Data Availability
Data used for this study were based on the MajesTEC-1, LocoMMotion, and MoMMent studies. MajesTEC-1 data sharing is governed by the Janssen Pharmaceutical Companies of Johnson & Johnson data sharing policy that is available online. As noted in the policy, requests for access to the study data can be submitted through Yale Open Data.
References
Tanenbaum B, Miett T, Patel SA. The emerging therapeutic landscape of relapsed/refractory multiple myeloma. Ann Hematol. 2023;102(1):1–11. https://doi.org/10.1007/s00277-022-05058-5.
Franssen LE, Mutis T, Lokhorst HM, van de Donk N. Immunotherapy in myeloma: how far have we come? Ther Adv Hematol. 2019;10:2040620718822660. https://doi.org/10.1177/2040620718822660.
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Hemasphere. 2021;5(2):e528. https://doi.org/10.1097/hs9.0000000000000528.
Ravi P, Kumar SK, Cerhan JR, et al. Defining cure in multiple myeloma: a comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018;8(3):26. https://doi.org/10.1038/s41408-018-0065-8.
Ramasamy K, Gay F, Weisel K, Zweegman S, Mateos MV, Richardson P. Improving outcomes for patients with relapsed multiple myeloma: challenges and considerations of current and emerging treatment options. Blood Rev. 2021;49:100808. https://doi.org/10.1016/j.blre.2021.100808.
Fonseca R, Usmani SZ, Mehra M, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20(1):1087. https://doi.org/10.1186/s12885-020-07503-y.
Mateos MV, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. 2022;36(5):1371–6. https://doi.org/10.1038/s41375-022-01531-2.
Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–75. https://doi.org/10.1038/s41375-019-0435-7.
ABECMA (idecabtagene vicleucel). Package insert. Bristol-Myers Squibb Company; 2021.
ABECMA (idecabtagene vicleucel). Summary of product characteristics. Celgene Distribution BV; 2021.
CARVYKTI (ciltacabtagene autoleucel). Janssen Biologics BV; 2022.
CARVYKTI (ciltacabtagene autoleucel). Package insert. Janssen Biotech, Inc.; 2022.
Martin T, Usmani SZ, Berdeja JG, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41(6):1265–74. https://doi.org/10.1200/jco.22.00842.
Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16. https://doi.org/10.1056/NEJMoa2024850.
Moreau P, Garfall AL, van de Donk N, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495–505. https://doi.org/10.1056/NEJMoa2203478.
Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232–44. https://doi.org/10.1056/NEJMoa2204591.
Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29(9):2259–67. https://doi.org/10.1038/s41591-023-02528-9.
TECVAYLI (teclistamab-cqyv). Package insert. Janssen Biotech, Inc; 2022.
TECVAYLI (teclistamab). Summary of product characteristics. Janssen Biologics BV; 2022.
ELREXFIO (elranatamab-bcmm). Package insert. Pfizer, Inc; 2023.
TALVEY (talquetamab-tgvs). Package insert. Janssen Biotech, Inc; 2023.
TALVEY (talquetamab). Summary of product characteristics. Janssen Biologics BV; 2023.
NEXPOVIO (selinexor). Summary of product characteristics. Stemline Therapeutics BV; 2021.
XPOVIO (selinexor). Package insert. Karyopharm Therapeutics; 2019.
Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–38. https://doi.org/10.1056/NEJMoa1903455.
van de Donk NWCJ. Long-term follow-up from MajesTEC-1 of teclistamab, a BCMA×CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. The 2023 American Society of Clinical Oncology (ASCO) Annual Meeting; June 2–6, 2023; Chicago, IL, USA & Virtual.
Moreau P, van de Donk N, Delforge M, et al. Comparative efficacy of teclistamab versus current treatments in real-world clinical practice in the prospective locommotion study in patients with triple-class-exposed relapsed and/or refractory multiple myeloma. Adv Ther. 2023;40(5):2412–25. https://doi.org/10.1007/s12325-023-02480-7.
Durie BGM, Kumar SK, Usmani SZ, et al. Daratumumab-lenalidomide-dexamethasone vs standard-of-care regimens: efficacy in transplant-ineligible untreated myeloma. Am J Hematol. 2020;95(12):1486–94. https://doi.org/10.1002/ajh.25963.
Mateos MV. Comparative effectiveness of teclistamab vs real-world physician’s choice of therapy in the locomotion and moment studies for patients with triple-class exposed relapsed/refractory multiple myeloma. In: Presented at the 20th International Myeloma Society (IMS) Annual Meeting; September 27–30, 2023; Athens, Greece.
Weisel K. Standard of care outcomes in the last 3 years in patients with triple-class exposed relapsed/refractory multiple myeloma: the first pooled analysis of LocoMMotion and MoMMent Trials. The 20th International Myeloma Society (IMS) Annual Meeting; September 27–30, 2023; Athens, Greece.
Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–46. https://doi.org/10.1016/s1470-2045(16)30206-6.
Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390–400. https://doi.org/10.1080/01621459.2016.1260466.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. https://doi.org/10.1002/sim.3697.
Moreau P. LocoMMotion: a prospective, observational, multinational study of real-life current standards of care in patients with relapsed/refractory multiple myeloma—final analysis at 2-year follow-up. In: Presented at the European Hematology Association (EHA) 2023 Hybrid Congress; June 8–11, 2023; Frankfurt, Germany.
Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–28. https://doi.org/10.1016/j.jval.2011.04.002.
Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Mak. 2018;38(2):200–11. https://doi.org/10.1177/0272989x17725740.
Ravi G, Costa LJ. Bispecific T-cell engagers for treatment of multiple myeloma. Am J Hematol. 2023;98(Suppl 2):S13–S21. https://doi.org/10.1002/ajh.26628.
Myeloma UK Selinexor Horizons Infosheet. https://www.myeloma.org.uk/library/selinexor-infosheet/. Accessed Aug 7, 2023.
BLENREP (belantamab mafodotin-blmf). Summary of product characteristics. GlaxoSmithKline Manufacturing SpA; 2020.
GSK provides an update on Blenrep (belantamab mafodotin-blmf) US marketing authorisation. 2022. https://www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-blenrep-us-marketing-authorisation. Accessed Sept 20, 2023.
EMA recommends non-renewal of authorisation of multiple myeloma medicine Blenrep. 2023. https://www.ema.europa.eu/en/news/ema-recommends-non-renewal-authorisation-multiple-myeloma-medicine-blenrep. Accessed Sept 19, 2023.
Kourelis T, Bansal R, Berdeja J, et al. Ethical challenges with multiple myeloma BCMA chimeric antigen receptor T cell slot allocation: a multi-institution experience. Transplant Cell Ther. 2023;29(4):255–8. https://doi.org/10.1016/j.jtct.2023.01.012.
Acknowledgements
We thank the patients who volunteered to participate in the study, their families and caregivers, the physicians and nurses who cared for the patients and supported this clinical trial, and staff members involved in data collection and analysis.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Kristin M. Allan, PhD, of Eloquent Scientific Solutions, and funded by Janssen Global Services, LLC.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
Open Access funding enabled and organized by Projekt DEAL. MajesTEC-1 and MoMMent were funded by Janssen Research & Development, LLC. LocoMMotion was funded by Janssen Research & Development, LLC, and Legend Biotech, Inc. Funding for the publication of this study, including the journal’s Rapid Service and Open Access fees, was provided by Janssen Global Services, LLC.
Author information
Authors and Affiliations
Contributions
Philippe Moreau: data collection, manuscript review. María-Victoria Mateos: data collection, manuscript review. Maria Esther Gonzalez Garcia: data collection, manuscript review. Hermann Einsele: data collection, manuscript review. Valerio De Stefano: data collection, manuscript review. Lionel Karlin: data collection, manuscript review. Joanne Lindsey-Hill: data collection, manuscript review. Britta Besemer: data collection, manuscript review. Laure Vincent: data collection, manuscript review. Suriya Kirkpatrick: data collection, manuscript review. Michel Delforge: data collection, manuscript review. Aurore Perrot: data collection, manuscript review. Niels W.C.J. van de Donk: data collection, manuscript review. Charlotte Pawlyn: data collection, manuscript review. Salomon Manier: data collection, manuscript review. Xavier Leleu: data collection, manuscript review. Joaquin Martinez-Lopez: data collection, manuscript review. Francesca Ghilotti: methodology, data analysis, interpretation, visualization, manuscript review. Joris Diels: methodology, data analysis, interpretation, visualization, manuscript review. Raúl Morano: study design, data analysis, interpretation, manuscript review. Claire Albrecht: study design, conceptualization, analysis, supervision, manuscript review. Vadim Strulev: study design, data analysis, interpretation, manuscript review. Imène Haddad: study design, data analysis, interpretation, manuscript review. Lixia Pei: methodology, data analysis, interpretation, visualization, manuscript review. Rachel Kobos: study design, data analysis, interpretation, manuscript review. Jennifer Smit: study design, data analysis, interpretation, manuscript review. Mary Slavcev: study design, data analysis, interpretation, manuscript review. Alexander Marshall: study design, data analysis, interpretation, manuscript review. Katja Weisel: data collection, manuscript review.
Corresponding author
Ethics declarations
Conflict of Interest
Philippe Moreau: served in a consulting/advisory role and has received honoraria from AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen, Oncopeptides and Sanofi. María-Victoria Mateos: served in a consulting/advisory role for AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Pfizer, Regeneron, Roche/Genentech, and Takeda; and has received honoraria from AbbVie/Genentech, Amgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Sanofi and Takeda. Maria Esther Gonzalez Garcia: no conflicts of interest to disclose. Hermann Einsele: received research funding or honoraria from and has served as a consultant or adviser for Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Janssen and Sanofi. Valerio De Stefano: served on an advisory board or speakers’ bureau and received honoraria from AbbVie, Alexion, Amgen, AOP Health, Argenx, Bristol Myers Squibb, Grifols, GlaxoSmithKline, Leo Pharma, Novartis, Novo Nordisk, Sanofi, SOBI and Takeda. Lionel Karlin: has served in a consulting/advisory role for Amgen, Janssen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Sanofi, Stemline, Takeda and AbbVie; received honoraria from AbbVie, Amgen, Celgene/Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, Stemline and Takeda; has received travel expenses from Amgen, Celgene/Bristol Myers Squibb, Janssen, Sanofi and Takeda; and has an immediate family member employed by Laboratoire Aguettant. Joanne Lindsey-Hill: has received consulting fees from AbbVie and received travel expenses from Janssen. Britta Besemer: received travel, accommodations, and expenses and received honoraria from Amgen, GlaxoSmithKline and Janssen-Cilag. Laure Vincent: received funding from Janssen; received honoraria for consulting/advisory role from Bristol Myers Squibb and Janssen; received travel expenses from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi and Takeda; and has participated in a Data Safety Monitoring Board or Advisory Board for Bristol Myers Squibb, Janssen and Takeda. Suriya Kirkpatrick: received honoraria from Celgene/Bristol Myers Squibb; received travel expenses from AbbVie, Celgene/Bristol Myers Squibb and Janssen; participated in a Data Safety Monitoring Board or Advisory Board for Celgene; and served in a leadership or fiduciary role for UKONS Research MIG and UK Lung Cancer Nurses RIG. Michel Delforge: served in a consulting/advisory role for Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, Stemline and Takeda; and has received research funding from Janssen. Aurore Perrot: served in a consulting role and received honoraria from AbbVie, Amgen, Celgene, Janssen-Cilag, Pfizer, Sanofi and Takeda. Niels W.C.J. van de Donk: served in a consulting/advisory role for AbbVie, Adaptive Biotechnologies, Amgen, Bayer, Bristol Myers Squibb, Celgene, Janssen, Novartis, Pfizer, Roche, Servier and Takeda; and has received research funding from Amgen, Bristol Myers Squibb, Celgene, Cellectis, Janssen and Novartis. Charlotte Pawlyn: received honoraria from AbbVie, Amgen, Celgene/Bristol Myers Squibb, Janssen, Pfizer, Sanofi and Takeda. Salomon Manier: received research funding from AbbVie, Celgene/Bristol Myers Squibb, Janssen, Novartis and Takeda. Xavier Leleu: held a consulting or advisory role for AbbVie, Amgen, Bristol Myers Squibb, CARsgen, Celgene, Gilead, GlaxoSmithKline, Janssen-Cilag, Karyopharm, Merck, Novartis, Oncopeptides, Roche and Takeda; received travel, accommodations and/or expenses from Takeda; and received honoraria from AbbVie, Amgen, Bristol Myers Squibb, CARsgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm, Merck, Novartis, Oncopeptides, Roche, Sanofi and Takeda. Joaquin Martinez-Lopez: has served in a consulting or advisory role, received speakers’ bureau fees and research funding from Bristol Myers Squibb, Janssen and Novartis. Francesca Ghilotti, Joris Diels, Raúl Morano, Imène Haddad, Lixia Pei, Rachel Kobos, Jennifer Smit, Mary Slavcev and Alexander Marshall: employed by Janssen. Claire Albrecht and Vadim Strulev: employed by and own stock in Janssen. Katja Weisel: served as a consultant for and received honoraria from Adaptive Biotechnologies, Karyopharm and Takeda; has received honoraria from Roche; and has received honoraria and served as a member on boards of directors and/or advisory committees for Amgen, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Oncopeptide and Sanofi.
Ethical Approval
Data analyzed in this study were received from the MajesTEC-1, LocoMMotion, and MoMMent studies. MajesTEC-1 was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent. An independent ethics committee or institutional review board at each study center approved the study protocol. The LocoMMotion and MoMMent studies were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. An independent ethics committee/institutional review board at each center approved the study protocol, listed in the Supplementary Material.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Moreau, P., Mateos, MV., Gonzalez Garcia, M.E. et al. Comparative Effectiveness of Teclistamab Versus Real-World Physician’s Choice of Therapy in LocoMMotion and MoMMent in Triple-Class Exposed Relapsed/Refractory Multiple Myeloma. Adv Ther 41, 696–715 (2024). https://doi.org/10.1007/s12325-023-02738-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02738-0