Abstract
Introduction
Fosnetupitant is a novel neurokinin 1 receptor antagonist (NK1RA) with favorable antiemetic efficacy in patients receiving emetogenic chemotherapy. This study assessed the efficacy of fosnetupitant in combination with palonosetron and dexamethasone and identified risk factors for chemotherapy-induced nausea and vomiting (CINV) for up to 168 h after treatment using pooled data from Japanese studies.
Methods
A pooled analysis of randomized phase II and phase III studies was performed to compare the efficacy of fosnetupitant and fosaprepitant in patients receiving cisplatin-based chemotherapy. The complete response (CR; no vomiting and no rescue medication) rate, CINV risk factors in various phases (0–120, 0–168, and 120–168 h), and impact of the number of risk factors on the time to treatment failure (TTF) were examined in the overall and NK1RA evaluable populations.
Results
In the combined cohort of NK1RA evaluable patients (n = 980), the CR rate at 0–168 h was significantly better in the fosnetupitant 235 mg group than in the fosaprepitant group (rate difference = 6.8%, 95% confidence interval = 1.0–12.7, p = 0.022). In the overall (n = 1368) and NK1RA evaluable populations, the CINV risk factor at 120–168 h was treatment failure in the first 120 h. TTF deteriorated as the number of identified CINV risk factors increased.
Conclusion
This analysis revealed that fosnetupitant could have long-acting antiemetic potency (> 120 h) and indicated the importance of antiemetic therapy at 0–120 h for CINV up to 168 h after chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Fosnetupitant can be used prophylactically as an antiemetic for chemotherapy-induced nausea and vomiting (CINV); however, its longer-term efficacy needs to be confirmed. |
The risk factors for CINV up to 168 h after chemotherapy administration are unclear. |
What was learned from this study? |
The complete response rate was significantly better with fosnetupitant compared with fosaprepitant. |
Treatment failure in the first 120 h after chemotherapy administration was a risk factor for CINV. |
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a frequent adverse event of anticancer drugs [1,2,3,4,5]. There are reports that the development of CINV negatively affects the quality of life (QOL) of patients with cancer [6, 7]. Therefore, the prevention and management of CINV are crucial for the continuation of chemotherapy and maintaining QOL.
For highly emetogenic chemotherapy (HEC), which causes CINV in > 90% of patients who do not receive any prophylaxis, and moderately emetogenic chemotherapy (MEC), which causes CINV in 30–90% of patients, current guidelines recommend proactive preventive antiemetic therapy. For patients receiving HEC or certain MEC regimens, three-drug combination therapy with a neurokinin 1 (NK1) receptor antagonist, a serotonin (5HT3) receptor antagonist, and dexamethasone (DEX) is mainly used, and in some guidelines, four-drug combination therapy featuring the addition of olanzapine is recommended or considered an option [8,9,10,11]. The timing of CINV onset is classified as acute (within 24 h after chemotherapy initiation) or delayed (> 24 h after chemotherapy initiation). Although most clinical trials assessed the occurrence of CINV events up to 5 days, delayed CINV often occurs even after 5 days. In fact, a large prospective observational study in Japan revealed that 15–25% of patients with cancer who received cisplatin-based HEC, non-cisplatin–based HEC, or MEC had nausea on days 6 and 7 [12]. To maintain patient QOL, it is important to consider antiemetic prophylaxis based on the premise that CINV develops during the conventional overall phase (0–120 h after anticancer drug administration) and the longer period up to at least 7 days.
Although the risk of CINV depends heavily on the type of anticancer drug, patient-related factors must also be considered. Patient-related risk factors described in various guidelines include young age, female sex, and drinking history [8, 9]. However, studies that identified these patient-related risk factors contained a variety of anticancer drug regimens and antiemetic regimens. Patient-related risk factors in cisplatin-containing chemotherapy with NK1 receptor antagonist (NK1RA) have not been clearly identified.
In two studies that evaluated fosnetupitant (FosNTP), a novel NK1RA, in patients receiving cisplatin-based chemotherapy, CINV events were evaluated for up to 168 h after cisplatin administration [13,14,15]. Although FosNTP tended to improve CINV control at 0–168 h, there is not enough evidence of its antiemetic efficacy. In addition, CINV risk factors for cisplatin-based chemotherapy at 0–168 h have not been clearly extracted. Thus, using the datasets of two studies that evaluated FosNTP in patients receiving a cisplatin-based regimen for 7 days, we assessed the efficacy of FosNTP in combination with the 5HT3 receptor antagonist palonosetron (PALO) and DEX for up to 168 h after treatment and investigated general or each drug’s risk factors for CINV in the overall population or the patients who received FosNTP or fosaprepitant (FosAPR), respectively.
Methods
Study Design and Treatment
The datasets of two studies that evaluated the efficacy and safety of FosNTP in patients with cancer who had been scheduled to receive cisplatin-based anticancer therapy were combined to form the dataset for this study. The main eligibility criteria common to both studies were age ≥ 20 years at the time of enrollment, receipt of an anticancer drug regimen containing ≥ 70 mg/m2 cisplatin, and provision of consent to participate [13, 14]. The phase II study was designed to evaluate the antiemetic effect and safety of FosNTP 81 and 235 mg in combination with PALO 0.75 mg and DEX versus placebo. The phase III study (CONSOLE study) examined the antiemetic effect and safety of FosAPR 150 mg and FosNTP 235 mg, both in combination with PALO 0.75 mg and DEX. From the CONSOLE study, we utilized a dataset regarding a single chemotherapy cycle, which was the first cycle of anticancer drugs during which the antiemetic effects of FosNTP and FosAPR on CINV were investigated. The primary endpoint of both studies was the overall complete response (CR; no emetic event and no rescue medication) rate.
In this analysis, the overall population was defined as all patients in the FosNTP 81 mg, FosNTP 235 mg, FosAPR, and placebo groups. The population combining the FosNTP 235 mg and FosAPR groups comprised the NK1RA evaluable population. Because FosNTP 81 mg is not approved in Japan, the FosNTP 81 mg group was excluded from the NK1RA evaluable population. Efficacy endpoints in the acute (0–24 h), delayed (24–120 h), overall (0–120 h), extended overall (0–168 h), extended delayed (24–168 h), and beyond delayed (120–168 h) phases were examined.
Statistical Analysis
The analysis set comprised the full analysis sets (patient populations to which cisplatin, PALO, DEX, and study drugs, or placebo was administered on the first day of anticancer drug administration) of both studies [13, 14].
To explore risk factors, we selected the following patient background factors in advance for analysis: age (< 55 years/ ≥ 55 years), sex (male/female), Eastern Cooperative Oncology Group performance status (0/1), drinking history (no or rarely/yes), smoking history (no/yes), motion sickness (no/yes), pregnancy-associated vomiting (no/yes), type of cancer (lung/other), cisplatin dose (< 80 mg/m2/ ≥ 80 mg/m2), NK1RA (FosNTP 81 mg/FosNTP 235 mg/FosAPR/placebo), and treatment failure in 0–120 h (no/yes).
The CR, total control (TC), and no nausea rates in each phase were calculated in each population and each study, and differences in treatment outcomes between the FosNTP 235 mg and FosAPR groups and their 95% confidence intervals (CIs) were calculated. The two groups were compared using Fisher’s exact test.
In risk factor analysis, univariate and multivariate logistic regression were performed for the overall population and NK1RA evaluable population with treatment failure (no CR) in each phase as a response variable and the aforementioned patient background factors as explanatory variables. The odds ratio, 95% CI, and p-value for each background factor were calculated. A background factor, namely treatment failure in 0–120 h (no/yes), was included as an explanatory variable only when risk factors for treatment failure in 120–168 h were investigated. For multivariate logistic regression, a full model and the backward stepwise procedure were applied. Background factors significant at p < 0.05 using the backward stepwise procedure were identified as risk factors. To evaluate the association between the number of risk factors identified and treatment failure, the Cochran-Armitage trend test was performed. Moreover, the time to treatment failure (TTF; time to the first emetic event or the use of rescue medication) was estimated according to the number of risk factors using the Kaplan-Meier method. For intergroup comparisons, the log-rank test was performed.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Compliance with Ethics Guidelines
This research was a pooled analysis of data obtained from two previous studies. Both previous studies were performed in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the protocols were approved by the institutional review board of Hamamatsu University Hospital (Hamamatsu-638 and -688) and other participating institutions (Table S1).
Informed consent was obtained from all individual participants included in the two previous studies from which data were pooled and analyzed in the present study.
Results
Patient Backgrounds in the Combined Dataset
The numbers of analyzed patients in the overall and NK1 evaluable populations were 1368 and 980, respectively. Of these patients, 587 (n = 195 in the phase II study and n = 392 in the phase III study) were included in the FosNTP 235 mg group. Patient characteristics in each group are presented in detail in Table S2. Among the treatment groups, no large imbalance in patient background factors was observed.
Efficacy in the Combined Dataset
The CR, TC, and no nausea rates in the FosNTP 235 mg and FosAPR groups in each phase are presented in Table 1. In the overall phase, the CR, TC, and no nausea rates did not differ between the FosNTP 235 mg and FosAPR groups. The CR rates during the extended overall phase (0–168 h) were 73.8% and 66.9% in the FosNTP 235 mg and FosAPR groups, respectively, with a rate difference of 6.8% (95% CI 1.0–12.7, p = 0.022). The CR rates in these groups during the extended delayed phase (24–168 h) were 74.8% and 68.4%, respectively, with a rate difference of 6.3% (95% CI 0.6–12.1, p = 0.035). The CR rates during the beyond delayed phase (120–168 h) were 86.7% and 81.4% in the FosNTP 235 mg and FosAPR groups, respectively, with a rate difference of 5.3% (95% CI 0.6–10.0, p = 0.030).
Exploration of Risk Factors Among Patients in the Overall Population
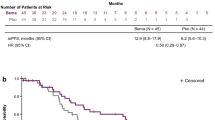
The results of the exploration of contributing factors to treatment failure in the overall population are presented in Table S3 and summarized in Table 2. The following CINV risk factors were identified in the overall phase: sex (female), performance status (1), drinking history (no or rarely), smoking history (no), motion sickness (yes), and NK1RA (placebo). The CINV risk factors during the extended overall phase were as follows: sex (female), performance status (1), drinking history (no or rarely), smoking history (no), and NK1RA (placebo). Meanwhile, only treatment failure at 0–120 h (yes) was identified as a CINV risk factor during the beyond delayed phase. Moreover, a significant correlation was observed between the number of identified risk factors and treatment failure (Table 3). In addition, TTF tended to deteriorate as the number of risk factors increased (Fig. 1).
Exploration of Risk Factors in the NK1RA Evaluable Population
The results of the exploration of contributing factors to treatment failure in the NK1RA evaluable population are presented in Table S4 and summarized in Table 2. In the FosNTP 235 mg group, the following CINV risk factors were identified in the overall phase: drinking history (no or rarely), smoking history (no), and motion sickness (yes). The risk factors during the extended overall phase were drinking history (no or rarely) and smoking history (no), and the only identified risk factor during the beyond delayed phase was treatment failure in 0–120 h (yes). Conversely, in the FosAPR group, the CINV risk factors in the overall phase were smoking history (no) and motion sickness (yes), whereas the risk factors during the extended overall phase and beyond delayed phase were sex (female) and treatment failure in 0–120 h (yes), respectively. In both treatment groups, a significant correlation was observed between the number of risk factors identified and treatment failure during the extended overall and beyond delayed phases (Table 4). In addition, TTF became significantly shorter as the number of risk factors increased (Fig. 2a, b). The estimated TTFs for FosNTP 235 mg and FosAPR among patients with zero risk factors or one or more risk factors are presented in Figure S1.
Discussion
In the present analysis, we combined the datasets of two studies that evaluated the role of FosNTP in patients receiving cisplatin-based chemotherapy, which is an HEC, to further confirm the efficacy of antiemetic therapy with FosNTP and explored CINV risk factors in various phases [13, 14]. The CR rate at 0–168 h (extended overall phase) was significantly higher for FosNTP than for FosAPR. In the overall and NK1RA evaluable populations, treatment failure in the first 120 h was related to the risk of CINV events at 120–168 h.
This analysis used data from two clinical studies with longer observation periods up to 168 h. One study was a phase II trial with 594 patients that assessed the efficacy and safety of FosNTP combined with PALO and DEX for the prevention of CINV in Japanese patients receiving cisplatin-based chemotherapy. The FosNTP dose of 235 mg was found to be more effective than placebo and FosNTP 81 mg. In the confirmatory phase III CONSOLE study with 795 patients, the overall CR rates were 75.2% and 71.0% in the FosNTP 235 mg and FosAPR groups, respectively, demonstrating the non-inferiority of FosNTP to FosAPR. The CR, TC, and no nausea rates tended to be higher for antiemetic therapy with FosNTP than for FosAPR in the extended overall and beyond delayed phases, but the differences were not statistically significant. When the data from these two studies with 980 patients were pooled and analyzed, the CR rate was significantly higher in the FosNTP 235 mg group than in the FosAPR group in longer periods up to 168 h (0–168, 24–168, and 120–168 h). It is assumed that the longer plasma half-life of netupitant, the active form of FosNTP (70 h for netupitant [13] vs. 9–13 h for the active form of FosAPR [16]) enables it to maintain its efficacy for a longer time including the beyond delayed phase [13]. Conversely, the differences in the CR rate in the evaluation periods including 168 h ranged 5.3–6.8% in this study, which were lower than the clinically meaningful threshold of 10% as indicated by MASCC and ESMO guideline panel members [17]; therefore, further confirmatory investigation is warranted. A relatively large number of patients experience CINV after 120 h, which indicates the necessity to evaluate antiemetic drugs during a longer period. Therefore, this result represents a potential unmet medical need that has not been observed in previous clinical trials for antiemetic therapy. The persistence of antiemetic efficacy for longer periods, which was made possible by triple therapy with FosNTP, PALO, and DEX, might provide better antiemetic control in these patients.
In this study, we explored the risk factors for the occurrence of nausea and vomiting in patients receiving cisplatin-based chemotherapy. This analysis has two novel features. One feature is that this study investigated risk factors over a longer period than previous studies, and another is that it explored risk factors separately according to the type of NK1RA, FosNTP 235 mg and FosAPR. In this analysis, no large difference was observed in CINV risk factors between the overall and extended overall phases. In the beyond delayed phase, treatment failure in the overall phase was extracted as a CINV risk factor. This finding highlighted the necessity of proactive antiemetic therapy from the beginning of the anticancer therapy to improve antiemetic control in the later phase. Usually, anticipatory CINV is connected with the CINV events that occurred during previous chemotherapy [18,19,20]. The poor antiemetic control might affect CINV control in the later phase in the identical course [21].
When the analysis of CINV risk factors in the extended overall phase was performed in the FosNTP 235 mg and FosAPR groups separately, drinking history (no or rarely) and smoking history (no) were identified as risk factors for the former group, and sex (female) was identified as a risk factor for the latter group. Regarding the difference in risk factors between the two groups, there might be interactions among these factors. In univariate analysis, drinking history (no or rarely), smoking history (no), and sex (female) had significant odds ratios in both groups. Because of their strong associations with CINV and the limited number of cases, it is possible that multivariate analysis did not simultaneously identify them as risk factors. The importance of antiemetic control in the overall phase against the beyond delayed phase was also confirmed in both the FosNTP 235 mg and FosAPR groups.
In the overall and NK1RA evaluable populations, a significant correlation between the number of CINV risk factors and TTF was observed. It is important to implement antiemetic therapy in consideration of the increased risk of CINV. If necessary, additional antiemetics such as olanzapine should be added. The addition of olanzapine 5 or 10 mg to triple antiemetic therapy improves antiemetic control for cisplatin-based chemotherapy [22, 23]. Because the combination of FosNTP and olanzapine has not been examined in clinical trials, future studies are warranted to confirm the superiority of adding olanzapine to FosNTP-containing antiemetic therapy.
The present analysis had some limitations. The first was that intrinsic CINV risk factors other than the predetermined patient background factors could exist. Although the background factors investigated in this analysis included those described by guidelines, a different report also listed the use of non-prescribed antiemetics at home and < 7 h of sleep as CINV risk factors [24]. Most patients analyzed in this study had non-small-cell lung cancer, and the outcome of antiemetic therapy can vary depending on the type of platinum-containing regimens (CR rate during the 0–168-h period: carboplatin + etoposide, 77%; carboplatin + paclitaxel, 67%; and carboplatin + pemetrexed, 54%) [25]. In the CONSOLE study, various anticancer drugs were used concurrently with cisplatin, and the efficacy and risk factors of individual regimens have not been investigated [14]. The second limitation was generalizability because the analysis consisted of Japanese individuals. Because racial differences in CINV risk have not yet been reported, the present results can be extrapolated to races other than Japanese. Conversely, as previously described, there may be unknown or intrinsic CINV risk factors. The dose of PALO varies between other countries and Japan (0.25 and 0.75 mg, respectively), and it was reported that the efficacy of PALO did not differ between different doses [26]. Lastly, it is unclear whether the present investigation included an adequate number of patients to identify CINV risk factors.
Conclusion
In conclusion, a pooled analysis of two studies showed the CR rates at the 0–168, 24–168, and 120–168 h (extended overall, extended delayed, and beyond delayed phases, respectively) were higher for FosNTP than for FosAPR. Treatment failure in the first 120 h was identified as a risk factor for CINV at 120–168 h. Additionally, significant correlations between TTF and CINV risk factors were found in these phases.
Data Availability
The data underlying this article are not publicly available because of the sponsor’s disclosure policy. The sponsor’s policy on data sharing may be found at https://www.taiho.co.jp/en/science/policy/clinical_trial_information_disclosure_policy/index.html.
References
Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13:219–27. https://doi.org/10.1007/s00520-004-0710-6.
Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142:101–7. https://doi.org/10.1007/s10549-013-2727-3.
Gupta K, Walton R, Kataria SP. Chemotherapy-induced nausea and vomiting: pathogenesis, recommendations, and new trends. Cancer Treat Res Commun. 2021;26: 100278. https://doi.org/10.1016/j.ctarc.2020.100278.
Nozawa K, Shimizu C, Kakimoto M, et al. Quantitative assessment of appearance changes and related distress in cancer patients. Psychooncology. 2013;22:2140–7. https://doi.org/10.1002/pon.3268.
Aapro MS. Palonosetron as an anti-emetic and anti-nausea agent in oncology. Ther Clin Risk Manag. 2007;3:1009–20.
Salsman JM, Grunberg SM, Beaumont JL, et al. Communicating about chemotherapy-induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Canc Netw. 2012;10:149–57. https://doi.org/10.6004/jnccn.2012.0018.
Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol. 2016;99:13–36. https://doi.org/10.1016/j.critrevonc.2015.12.001.
Aogi K, Takeuchi H, Saeki T, et al. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis. Int J Clin Oncol. 2021;26:1–17. https://doi.org/10.1007/s10147-020-01818-3.
The National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Antiemesis. Version 1, 2023. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed May 17, 2023.
Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO guideline update. J Clin Oncol. 2020;38:2782–97. https://doi.org/10.1200/JCO.20.01296.
Multinational Association of Supportive Care in Cancer. MASCC/ESMO antiemetic guideline 2016 with updates in 2019. https://mascc.org/wp-content/uploads/2022/04/mascc_antiemetic_guidelines_english_v.1.5SEPT29.2019.pdf. Accessed Nov 11, 2022.
Tamura K, Aiba K, Saeki T, et al. Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol. 2015;20:855–65. https://doi.org/10.1007/s10147-015-0786-7.
Sugawara S, Inui N, Kanehara M, et al. Multicenter, placebo-controlled, double-blind, randomized study of fosnetupitant in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Cancer. 2019;125:4076–83. https://doi.org/10.1002/cncr.32429.
Hata A, Okamoto I, Inui N, et al. Randomized, double-blind, phase III study of fosnetupitant versus fosaprepitant for prevention of highly emetogenic chemotherapy-induced nausea and vomiting: CONSOLE. J Clin Oncol. 2022;40:180–8. https://doi.org/10.1200/JCO.21.01315.
Hata A, Shiraishi Y, Inui N, et al. Exploratory analysis comparing fosnetupitant versus fosaprepitant for prevention of highly emetogenic chemotherapy-induced nausea and vomiting (CINV): a randomized, double-blind, phase 3 study (CONSOLE). Oncol Ther. 2022;10:253–62. https://doi.org/10.1007/s40487-022-00188-2.
Merck & Co., Inc. Emend. Prescribing Information. 2008. Updated May 2022. https://www.merck.com/product/usa/pi_circulars/e/emend_iv/emend_iv_lowedta_pi.pdf. Accessed Sept 7, 2022.
Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119–33. https://doi.org/10.1093/annonc/mdw270.
Morrow GR, Morrell C. Behavioral treatment for the anticipatory nausea and vomiting induced by cancer chemotherapy. N Engl J Med. 1982;307:1476–80. https://doi.org/10.1056/NEJM198212093072402.
Morrow GR, Lindke J, Black PM. Predicting development of anticipatory nausea in cancer patients: prospective examination of eight clinical characteristics. J Pain Symptom Manag. 1991;6:215–23. https://doi.org/10.1016/0885-3924(91)90011-r.
Andrykowski MA, Jacobsen PB, Marks E, et al. Prevalence, predictors, and course of anticipatory nausea in women receiving adjuvant chemotherapy for breast cancer. Cancer. 1988;62:2607–13. https://doi.org/10.1002/1097-0142(19881215)62:12%3c2607::aid-cncr2820621226%3e3.0.co;2-s.
Lorusso V. Management of chemotherapy-induced nausea and vomiting by risk profile: role of netupitant/palonosetron. Ther Clin Risk Manag. 2016;12:917–25. https://doi.org/10.2147/TCRM.S89215.
Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134–42. https://doi.org/10.1056/NEJMoa1515725.
Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:242–9. https://doi.org/10.1016/S1470-2045(19)30678-3.
Dranitsaris G, Molassiotis A, Clemons M, et al. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28:1260–7. https://doi.org/10.1093/annonc/mdx100.
Matsui R, Suzuki K, Takiguchi T, et al. 5-Hydroxytryptamine-3 receptor antagonist and dexamethasone as prophylaxis for chemotherapy-induced nausea and vomiting during moderately emetic chemotherapy for solid tumors: a multicenter, prospective, observational study. BMC Pharmacol Toxicol. 2020;21:72. https://doi.org/10.1186/s40360-020-00445-y.
Likun Z, Xiang J, Yi B, Xin D, Tao ZL. A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist. 2011;16:207–16. https://doi.org/10.1634/theoncologist.2010-0198.
Medical Writing, Editorial, and Other Assistance
Data collection and analysis were supported by Taiho Pharmaceutical Co., Ltd. Translation and editorial assistance in the preparation of this article was provided by ASCA Corporation and funded by Taiho Pharmaceutical Co., Ltd.
Funding
This research, the journal’s Rapid Service and Open Access fees, and medical writing support were funded by Taiho Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, and all authors contributed to the manuscript writing. Material preparation and/or provision of patients were performed by Naoki Inui, Kaoru Kubota, Toshiaki Saeki, and Tomohide Tamura. Data collection were performed by Naoki Inui, Yukihiro Toi, Yasuto Yoneshima, Masahiro Morise, Akito Hata, and Tomohide Tamura. Data analysis and interpretation were performed by Naoki Inui. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Naoki Inui received honoraria and grants from Taiho Pharmaceutical. Yukihiro Toi received honoraria from Taiho Pharmaceutical, Bristol-Myers Squibb, Ono Pharmaceutical, MSD, AstraZeneca, Chugai Pharmaceutical, Pfizer, and Kyowa Kirin. Yasuto Yoneshima received honoraria from Taiho Pharmaceutical and Takeda Pharmaceutical. Masahiro Morise received funding from the speakers’ bureau of Chugai Pharmaceutical, AstraZeneca, Ono Pharmaceutical, and Eli Lilly; research funding from Boehringer Ingelheim (Inst), Novartis (Inst), AstraZeneca, (Inst), Eli Lilly (Inst), Taiho Pharmaceutical (Inst), Chugai Pharmaceutical (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Merck Serono (Inst), and Kissei Pharmaceutical (Inst). Akito Hata provided a consulting or advisory role to Boehringer Ingelheim, Eli Lilly, Chugai Pharmaceutical, AstraZeneca, and MSD; received funding from the speakers’ bureaus of Boehringer Ingelheim, Eli Lilly, Chugai Pharmaceutical, AstraZeneca, and Taiho Pharmaceutical; received research funding from Boehringer Ingelheim (Inst), MSD (Inst), Eli Lilly (Inst), and AstraZeneca (Inst). Kaoru Kubota received honoraria from Bristol-Myers Squibb Japan, Daiichi Sankyo, Boehringer Ingelheim, Taiho Pharmaceutical, Eli Lilly Japan, MSD, Chugai Pharmaceutical, AstraZeneca, Nippon Kayaku, Takeda Pharmaceutical, and Pfizer; and served on the advisory board for Taiho Pharmaceutical. Toshiaki Saeki received grants from AstraZeneca (Inst), Eisai (Inst), Kyowa Kirin (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), WJOG (Inst), Chugai Pharmaceutical (Inst), Nippon Kayaku (Inst), Novartis (Inst), MSD (Inst), Sawai Pharmaceutical (Inst), Covance Japan (Inst), Maruho (Inst), LabCorp Japan (Inst), Sanofi (Inst), Takeda Pharmaceutical (Inst), and Eli Lilly Japan (Inst); honoraria from Ono Pharmaceutical, Kyowa Kirin, Chugai Pharmaceutical, ASKA Pharmaceutical, Novartis, AstraZeneca, Eisai, Taiho Pharmaceutical, Takeda Pharmaceutical, Eli Lilly Japan, Pfizer Japan, MiRTeL, and Meiji Seika Pharma. Tomohide Tamura received honoraria from Chugai Pharmaceutical, Taiho Pharmaceutical, Boehringer Ingelheim, MSD, Ono Pharmaceutical, Eli Lilly, and Nippon Kayaku.
Ethical Approval
This research was a pooled analysis of data obtained from two previous studies. Both previous studies were performed in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the protocols were approved by the institutional review board of each participating institution. Informed consent was obtained from all individual participants included in the two previous studies from which data were pooled and analyzed in the present study. Consent for publication was not applicable, in our study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Inui, N., Toi, Y., Yoneshima, Y. et al. Pooled Analysis of Studies Evaluating Fosnetupitant and Risk Factors for Cisplatin-Induced Nausea and Vomiting During the Extended Overall Phase. Adv Ther 40, 4928–4944 (2023). https://doi.org/10.1007/s12325-023-02648-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02648-1