Abstract
Introduction
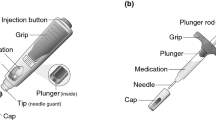
Pegfilgrastim-cbqv (UDENYCA®; Coherus BioSciences, Redwood City, CA, USA) is a pegfilgrastim (Neulasta®; Amgen, Thousand Oaks, CA, USA) biosimilar approved for administration by prefilled syringe (PFS). The recently approved pegfilgrastim-cbqv prefilled autoinjector (AI) was developed as another method of self-administration and to aid in-office use, providing flexibility in drug delivery. The objectives of the study were to assess the pharmacokinetics (PK) and pharmacodynamics (PD) to determine bioequivalence of the prefilled AI and the PFS for administration of pegfilgrastim-cbqv and to assess the safety profile of the prefilled AI.
Methods
During this open-label, two-period crossover study, healthy adult males (N = 155) were randomly assigned (1:1 ratio) to receive a subcutaneous injection of pegfilgrastim-cbqv using a prefilled AI (n = 76) or a PFS (n = 79) in period 1. During period 2, participants received an injection using the other method. Primary PK and secondary PD parameters were calculated to assess the bioequivalence of the treatment as administered by the two delivery methods. Safety and immunogenicity were also assessed.
Results
The 90% CIs of the geometric mean ratios for the PK and PD parameters were within the required range (80–125%), demonstrating bioequivalence between the pegfilgrastim-cbqv prefilled AI and PFS. Treatment-emergent adverse events (TEAEs) were reported by 75% and 74.1% of participants in the prefilled AI and PFS groups, respectively. The most common TEAEs in both treatment groups were myalgia, bone pain, and headache. AI-device–related TEAEs were injection site pain (1.4%) and injection site bruising (0.7%). The incidence of antidrug antibodies and neutralizing antibodies was low and was similar in both treatment sequences.
Conclusions
The bioequivalence of pegfilgrastim-cbqv administered using a prefilled AI and a PFS was established. The safety, including immunogenicity profiles, of pegfilgrastim-cbqv administered using the prefilled AI and the PFS were similar, with no new safety findings.
Graphical Abstract

Plain Language Summary
Pegfilgrastim-cbqv is used 24–96 h after chemotherapy to prevent febrile neutropenia. This is when a patient has a fever and a lower-than-normal number of white blood cells. Pegfilgrastim-cbqv is also used to treat someone who has a fever and a low number of white blood cells after high radiation exposure (hematopoietic acute radiation syndrome). Pegfilgrastim-cbqv is given as an injection by hand. The syringe is already filled with the drug. The US Food and Drug Administration also approved a prefilled autoinjector as another way to give the drug. The autoinjector is a spring-loaded system that delivers pegfilgrastim-cbqv in less than 10 s. A shield covers the needle before and after the drug is given. The autoinjector is safe and effective for patients to give pegfilgrastim-cbqv to themselves and for health care staff to use in-office. This study looked at whether the way in which pegfilgrastim-cbqv moves through the body (pharmacokinetics) and its effects on the body (pharmacodynamics) were similar in healthy adult males when using a prefilled autoinjector and a prefilled syringe. Side effects were also assessed. The two administration methods had very similar pharmacokinetic and pharmacodynamic results for pegfilgrastim-cbqv. The side effects for both groups were also similar. The most common side effects were muscle aches and pains, bone pain, and headaches. Giving pegfilgrastim-cbqv with the prefilled autoinjector was very similar to giving it with the prefilled syringe and the pharmacokinetics, pharmacodynamics, and safety were similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The pegfilgrastim biosimilar pegfilgrastim-cbqv (UDENYCA®; Coherus BioSciences) is administered using a prefilled syringe (PFS) to reduce the risk of infection that could result from febrile neutropenia after myelosuppressive chemotherapy and to treat hematopoietic subsyndrome of acute radiation syndrome (H-ARS) in patients acutely exposed to myelosuppressive doses of radiation. |
Administration of pegfilgrastim-cbqv using a prefilled autoinjector (AI) as an alternative to a PFS was safely and effectively self-administered, providing flexibility in drug delivery for in-home and in-office use; self-administration may eliminate additional visits to health care facilities. |
The study was conducted to establish the pharmacokinetics (PK) and pharmacodynamics (PD) to determine the bioequivalence of pegfilgrastim-cbqv administered using prefilled AI and PFS. It also was conducted to assess the safety profile (including immunogenicity) of pegfilgrastim-cbqv administered using the prefilled AI. |
What was learned from the study? |
The results of this study showed that PK, PD, and safety profiles of pegfilgrastim-cbqv, when administered using the prefilled AI, remain essentially unchanged compared with the PFS. These results contributed to the US Food and Drug Administration approval of the prefilled AI. |
The pegfilgrastim-cbqv prefilled AI is an alternative to the PFS method of administration, providing choice and flexibility to health care providers and patients in regard to dose delivery for in-office use and self-administration, reducing the need for administration at a clinic in the case of self-administration. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.23826333.
Introduction
Pegfilgrastim products are long-acting granulocyte colony-stimulating factor (G-CSF) biologics administered 24–96 h after chemotherapy for prophylaxis of infection that could result from febrile neutropenia (FN) [1,2,3,4,5]. FN is a serious yet common complication of myelosuppressive chemotherapy that is characterized by fever and a reduced absolute neutrophil count (ANC) of < 0.5 × 109/L [1]. Recently, the US Food and Drug Administration (FDA) approved pegfilgrastim-cbqv to treat patients who were exposed to myelosuppressive doses of radiation [2] and had hematopoietic subsyndrome of acute radiation syndrome (H-ARS), characterized by decreased bone marrow activity [6,7,8]. Patients with H-ARS receive the first dose of pegfilgrastim-cbqv as soon as possible after suspected or confirmed exposure to myelosuppressive doses of radiation and a second dose 1 week later [2, 7, 9].
Pegfilgrastim products are highly effective in preventing FN and treating H-ARS, but the treatment can be expensive. Biosimilars provide a less costly alternative to originator biologics (or reference products), such as pegfilgrastim [6, 7, 10, 11], while still being highly similar to the originator product in terms of potency, safety, and efficacy [12].
Pegfilgrastim-cbqv (UDENYCA®; Coherus BioSciences, Redwood City, CA, USA) is approved by the FDA as a biosimilar to the reference product pegfilgrastim (Neulasta®; Amgen, Thousand Oaks, CA, USA) [2, 3]. Bioequivalence between pegfilgrastim and pegfilgrastim-cbqv, as well as the safety (including immunogenicity) of pegfilgrastim-cbqv, was established by comparing the pharmacokinetics (PK) and pharmacodynamics (PD) in a randomized, single-blind study in healthy participants with administration of a 6-mg per 0.6-mL single-dose solution for injection using a prefilled syringe (PFS) [13].
Pegfilgrastim-cbqv is approved for injection using a PFS. Recently, a pegfilgrastim-cbqv 6-mg per 0.6-mL single-dose prefilled autoinjector (AI) drug-device combination was developed as an alternative and was approved by the FDA [2, 14]. Previous research has shown that patients prefer the use of an AI to a PFS because the anxiety often experienced by patients due to needle phobia may be minimized because the AI has no visible needle [15, 16]. Use of an AI has also provided safe and effective self-administration [15, 17] outside the clinic, and it may provide several potential advantages for in-office use within clinical practice. In addition, an AI offers patients and health care professionals flexibility in selecting the time and place of administration, whereas for an on-body injector the injection time is preset [18, 19]. Furthermore, because pegfilgrastim-cbqv is indicated for administration the day after chemotherapy for the prevention of FN and 1 week after the first dose of pegfilgrastim-cbqv for the treatment of H-ARS [5], it would be expected that self-administration using the prefilled AI would decrease the number of visits to health care facilities for the purpose of dose administration [10]. Finally, the use of an AI may provide a practical solution in situations that involve large numbers of patients with H-ARS after nuclear or radiologic disasters [7, 9].
The pegfilgrastim-cbqv AI product is composed of a 6-mg per 0.6-mL single-dose pegfilgrastim-cbqv solution contained in a naked prefilled syringe with AI components. The prefilled AI is an automated dose delivery system activated when the AI is pushed down onto the skin, delivering its contents in < 10 s [2, 20]. The pegfilgrastim-cbqv prefilled AI and PFS, as well as the reference product pegfilgrastim PFS, are identical in terms of dosage form, strength, route of administration, indication, and dosing regimen [2, 3].
As part of the license application, bioequivalence between the two methods of administration—PFS and AI—had to be established for the pegfilgrastim-cbqv prefilled AI to be approved by the FDA. Therefore, the primary objective of this study was to establish the PK bioequivalence of pegfilgrastim-cbqv administered using a prefilled AI and the PFS. The secondary objective was to assess the PD bioequivalence and characterize the safety profile (including immunogenicity) of pegfilgrastim-cbqv administered using the prefilled AI.
Methods
Study Design
This randomized, open-label, two-period, two-sequence, crossover study was conducted in four clinical sites in the United States over 14–16 weeks from 25 February to 10 November 2021. The study was carried out to assess the bioequivalence by evaluating PK and PD and safety (including immunogenicity) of a single 6-mg per 0.6-mL subcutaneous injection of pegfilgrastim-cbqv by a prefilled AI compared with a PFS.
Participants were randomly assigned on day 1 of period 1 to receive one of two treatment sequences in a 1:1 ratio: pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS or pegfilgrastim-cbqv PFS/pegfilgrastim-cbqv prefilled AI. After a washout period of 6–8 weeks, the assigned treatment was administered on day 1 of period 1, and then using the other treatment method on day 1 of period 2 (Supplemental Fig. S1).
To be eligible for period 2 dosing, an ANC of 1.7–7.2 × 103/mm3 and a white blood cell (WBC) count of 4.0–11.0 × 103/mm3 were required. These levels were assessed 2 days before period 2. Two subsequent WBC count measurements were permitted to allow the patient to meet the inclusion criteria, provided the prescribed washout period from period 1 dosing was not exceeded.
Participants were admitted to the clinical sites on day − 1 of period 1 and period 2 and discharged after 5 days. After admission, a single 6-mg per 0.6-mL dose of pegfilgrastim-cbqv was administered by prefilled AI or PFS on day 1. During admission, blood samples were collected at 30 min before dosing and at 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 60, 72, 84, and 96 h after dosing to assess the PK and PD parameters. After discharge, the participants returned to the clinical sites on days 6, 11, 13, 21, and on period 1, day 28 for blood sample collection. Anti-drug antibody (ADA) samples were collected on day 1 before dosing, on day 11 during periods 1 and 2, and on day 28, period 2 at the follow-up visit.
Participants and Treatment
The study population comprised healthy male participants 18–45 years of age with a body weight ≥ 50 and ≤ 100 kg and a body mass index between 18 and 28 kg/m2. Participants with any previous exposure to filgrastim or pegfilgrastim were excluded from the study. Pegfilgrastim-cbqv was provided as either a single-dose 6-mg per 0.6-mL PFS or as a prefilled AI. Pegfilgrastim-cbqv was administered at approximately the same time on day 1 of periods 1 and 2.
Ethical Approval
The Advarra Institutional Review Board reviewed and approved the protocol and informed consent form (IRB Registration Number: IRB0000971). All applicable laws and regulations were followed when conducting the study, including the International Conference for Harmonisation E6 Guideline on Good Clinical Practice. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
PK and PD End Points
A validated enzyme-linked immunosorbent assay (ELISA) was used to analyze pegfilgrastim-cbqv concentrations in the plasma samples. The assay was a modification of the Human G-CSF Quantikine ELISA kit (Bio-Techne, Minneapolis, MN, USA) and used anti–G-CSF antibodies as capture and detection reagents. The sensitivity (lower limit of quantification) of the assay in neat plasma was 150 pg/mL. All the study samples were analyzed within the validated stability limits for the assay, including temperature. The ANC analysis was performed by Clinical Laboratory Improvement Amendments certified laboratories utilizing FDA-regulated automated hematology analyzers, in which all analytical performance standards were evaluated and approved. PK and PD bioequivalence was calculated using Statistical Analysis System (SAS®; SAS Institute, Cary, NC) version 9.4.
The primary PK parameters were area under the concentration–time curve from time 0 to infinity (AUC0-inf), area under the concentration–time curve from time 0 to the last quantifiable concentration (AUC0-last), and maximum plasma concentration (Cmax). Additional PK parameters were time to Cmax, terminal elimination half-life, elimination rate constant, AUC from time 0 to 288 h, apparent extravascular clearance, volume of distribution, and percentage AUC extrapolated.
The key PD parameters were ANC time curve from time 0 to the last quantifiable ANC (ANC AUC0-last) and maximum ANC (ANCmax). Other PD parameters were time to maximum ANC and area under the ANC-time curve from time 0 to 480 h.
Safety
Assessment of the safety measures included evaluation of treatment-emergent adverse events (TEAEs), adverse events of special interest, serious adverse events (SAEs), local injection site reactions (ISRs), laboratory test results, hematology, coagulation, urinalysis results, and vital signs.
Immunogenicity
A validated electrochemiluminescence bridging assay was used to test samples for ADAs [21, 22]. Confirmed ADA-positive samples were characterized for titer and binding reactivity to polyethylene glycol (PEG) or G-CSF. To determine whether the ADAs were neutralizing, the ADA-positive samples were also tested using a validated cell-based neutralizing antibody (NAb) assay. NAb-positive samples underwent additional testing to determine their ability to neutralize endogenous G-CSF. Details of the immunogenicity assays (ADA and NAb) are discussed elsewhere [22].
Statistical Analysis
Determination of Sample Size
To support the primary objective of assessing bioequivalence, approximately 158 participants were randomly assigned 1:1 to each treatment sequence. These calculations were based on results from a previous pegfilgrastim-cbqv study [13], which assumed an intraparticipant percentage coefficient of variation (CV%) of pegfilgrastim-cbqv prefilled AI/PFS ≤ 47.5% for the primary PK end points, an expected true geometric mean ratio (GMR) of pegfilgrastim-cbqv prefilled AI/PFS ≤ 1.05 for the primary PK end points, and a dropout rate of 25%. The randomized population comprised all randomly assigned participants who received any amount of pegfilgrastim-cbqv using the PFS or prefilled AI during period 1.
Primary PK Bioequivalence Analysis
Using analysis of variance (ANOVA) that included terms of sequence, treatment group, and period as fixed effects and participant nested within sequence as a random effect, the logarithm-transformed parameters AUC0-inf, AUC0-last, and Cmax were analyzed to perform the PK bioequivalence assessment for the primary analysis of the PK-evaluable population. If the 90% CI for the GMRs of pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS fell within the prespecified range of 80–125% for AUC0-inf, AUC0-last, and Cmax, PK bioequivalence was considered established. The PK-evaluable population comprised all participants who had sufficient plasma concentration–time data to enable a reliable calculation of PK parameters for ≥ 1 of the primary PK end points and all participants who received 2 full doses of pegfilgrastim-cbqv.
Sensitivity Analysis for PK Bioequivalence Assessment
The robustness of the primary analysis was tested using a sensitivity analysis. The same ANOVA model used for the primary PK bioequivalence analysis was used to analyze the logarithm-transformed parameters AUC0-inf, AUC0-last, and Cmax. If the 90% CI for the GMR of pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS fell within the prespecified range of 80–125% for AUC0-inf, AUC0-last, and Cmax, PK bioequivalence was considered established.
For the sensitivity analysis, the PK-evaluable population and PK outliers were used. Any participant was considered a PK outlier if they exhibited an abnormally high or low PK response to one or the other of the products that was discordant with corresponding data for that participant or for the rest of the participants in the study. Mathematically, if a participant had ≥ 1 for the intraparticipant CV% between period 1 and period 2 that was > 100% for AUC0-inf, AUC0-last, and Cmax, that participant was considered a PK outlier.
Secondary PD Bioequivalence Analysis
Using the PD-evaluable population, PD parameters were summarized by treatment group and study period. The PD-evaluable population comprised all participants who had sufficient data to enable a reliable calculation of ≥ 1 primary PD parameter and participants who had received both full doses of pegfilgrastim-cbqv. Geometric mean and geometric CV% were added to the descriptive statistics.
Using an analysis of covariance model, including terms for treatment sequence, treatment group, and period as fixed effects, participant nested within sequence as a random effect and baseline ANC as a covariate, the logarithmic transformations of the PD parameters were analyzed for the PD-evaluable population in the formal PD bioequivalence assessment. If the 90% CI for the GMR of pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS fell within the prespecified range of 80–125% for ANC AUC0-last and ANCmax, PD bioequivalence was considered demonstrated.
Safety
Safety data were summarized and analyzed descriptively without formal statistical analysis. The safety of a pegfilgrastim-cbqv prefilled AI and of a PFS was evaluated using the safety population. The safety population comprised all participants who had been randomly assigned to one of the two treatment sequences and received one or more doses of pegfilgrastim-cbqv by prefilled AI or PFS.
Immunogenicity
Immunogenicity end points were the incidence and time course of ADAs, ADA titer, and NAbs. End points were summarized by treatment group or treatment-sequence group using the safety population. Additional analysis was done to evaluate the impact of ADAs or NAbs on PK, PD, and safety. The impact of immunogenicity on PK was evaluated using the PK concentration population, which consisted of all participants who had measurable plasma PK data after receiving any quantity of pegfilgrastim-cbqv. The impact of immunogenicity on PD and on safety was evaluated using the safety population.
Results
Participant Demographics and Treatment
Of the 155 participants included in the study, 76 were randomly assigned to the pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS treatment sequence and 79 to the pegfilgrastim-cbqv PFS/pegfilgrastim-cbqv prefilled AI treatment sequence. In total, 139 participants (89.7%) completed both study periods. As shown in Table 1, 16 participants (8 from each treatment sequence) withdrew early from the study. Participant disposition and characteristics were similar between both treatment sequences (Table 2).
Pharmacokinetics
In both treatment groups, the mean pegfilgrastim-cbqv plasma concentrations peaked at approximately 16–20 h in the PK-evaluable population, with a slow elimination thereafter. The mean plasma versus time curves for both treatment groups were similar at all time points (Fig. 1).
In the PK-evaluable population, the GMs for pegfilgrastim-cbqv prefilled AI and pegfilgrastim-cbqv PFS were 6972.0 h·ng/mL and 6656.6 h·ng/mL for AUC0-last, 7101.4 h·ng/mL and 6812.2 h·ng/mL for AUC0-inf, and 190.5 ng/mL and 177.6 ng/mL for Cmax (Supplementary Table S1).
The 90% CI of GMRs for AUC0-inf, AUC0-last, and Cmax were used to assess the PK bioequivalence of pegfilgrastim-cbqv administered by AI compared with the PFS. The GMR for AUC0-inf was 104.8 (90% CI 96.9–113.3), for AUC0-last was 105.3 (90% CI 97.7–113.5), and for Cmax was 107.8 (90% CI 100.1–116.1). PK bioequivalence was demonstrated between the pegfilgrastim-cbqv prefilled AI and the PFS in the PK-evaluable population because the 90% CIs for the GMRs for the PK parameters fell within the prespecified range (Table 3).
A sensitivity analysis was used to establish the robustness of the primary PK bioequivalence based on the GMRs of AUC0-inf, AUC0-last, and Cmax for the pegfilgrastim-cbqv prefilled AI compared with the PFS (participants were from the PK-evaluable population combined with the PK outliers). PK bioequivalence was shown by the sensitivity analysis as the 90% CIs for the GMRs of pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS were within the prespecified range (Table 4).
Pharmacodynamics
Both treatment groups showed a peak in the mean ANCs approximately 60 h after the dose. The ANC concentrations returned to baseline for both treatment groups by 480 h after dosing (Fig. 2). The calculated mean and highest ANCmax and time to maximum concentration (Tmax) values for the pegfilgrastim-cbqv prefilled AI and the pegfilgrastim-cbqv PFS were comparable (Supplementary Table S2). Overall, the mean ANC was similar between the two treatment groups over time.
The 90% CIs for the GMRs of ANC AUC0-last and ANCmax were used to assess the PD bioequivalence of pegfilgrastim-cbqv administered using a prefilled AI compared with a PFS. For the ANC AUC0-last, the GMR was 98.9 (90% CI 96.5–101.4), and for ANCmax, the GMR was 99.6 (90% CI 96.2–103.2). PD bioequivalence was shown in the PD-evaluable population because the 90% CIs for the GMRs of the pegfilgrastim-cbqv prefilled AI/pegfilgrastim-cbqv PFS were within the prespecified range of 80–125% (Supplementary Table S3).
Safety
The number of participants reporting ≥ 1 TEAE was 111 (75%) in the pegfilgrastim-cbqv prefilled AI group and 109 (74.1%) in the pegfilgrastim-cbqv PFS group; most AEs were mild to moderate. Only two participants experienced treatment-emergent serious adverse events (TESAEs) (one in the pegfilgrastim-cbqv prefilled AI group and one in the pegfilgrastim-cbqv PFS group), although neither was considered related to the study drug (Table 5). The participant in the pegfilgrastim-cbqv prefilled AI group was diagnosed with congenital absence of the right pulmonary artery on day 4 of period 2; the participant in the pegfilgrastim-cbqv PFS group experienced acute renal failure on day 23 of period 2.
TEAEs related to an ISR occurred in 12 participants (8.1%) in the pegfilgrastim-cbqv prefilled AI group and 13 participants (8.8%) in the pegfilgrastim-cbqv PFS group. Only one participant (0.7%) in the pegfilgrastim-cbqv prefilled AI group and no participants in the pegfilgrastim-cbqv PFS group withdrew from the study because of an AE deemed related to the study drug (Table 5). The participant who withdrew from the pegfilgrastim-cbqv prefilled AI group experienced left lower limb weakness and acute renal failure.
The most common study drug-related AEs were musculoskeletal and connective tissue disorders (53.4% vs. 53.1%), nervous system disorders (34.5% vs. 27.9%), and general disorders and administration site conditions (13.5% vs. 16.3%) for the pegfilgrastim-cbqv prefilled AI and the pegfilgrastim-cbqv PFS, respectively. TEAEs for the pegfilgrastim-cbqv prefilled AI and the pegfilgrastim-cbqv PFS were mainly those attributed to the expected musculoskeletal effects associated with the use of G-CSFs (Supplementary Table S4). Three participants (2.0%) experienced TEAEs related to the prefilled AI, of whom two participants (1.4%) reported injection site pain and one participant (0.7%) reported injection site bruising.
No clinically meaningful changes from baseline were observed in the median values for the chemistry or hematology laboratory tests. The safety laboratory results for chemistry, hematology, and urinalysis parameters showed no meaningful differences between treatment groups.
Immunogenicity
At baseline, 36 participants were positive for ADAs (Table 6). The number of treatment-emergent ADAs in period 1 was similar between participants who used the prefilled AI (37.7%) and those who used the PFS (33.9%). Both treatment groups had low ADA titers (Table 6) and were primarily directed to the PEG moiety of pegfilgrastim-cbqv. In addition, the overall incidence of ADAs was similar in both treatment sequences (Table 6).
NAbs were detected at baseline (before dosing) in three participants (two participants in the pegfilgrastim-cbqv prefilled AI treatment group and one in the pegfilgrastim-cbqv PFS treatment group). No NAbs were present in the post-dose samples.
Impact of Immunogenicity on PK and PD
The geometric means of Cmax and AUC values were compared in ADA-positive and ADA-negative participants based on treatment to determine the effect of ADAs on the PK of the pegfilgrastim-cbqv prefilled AI compared with the PFS. Based on treatment, the geometric means of Cmax and AUC values were similar in ADA-positive and ADA-negative participants (Supplementary Table S5). There was no impact of immunogenicity on the PK of the pegfilgrastim-cbqv prefilled AI or the PFS.
To determine the impact of immunogenicity on PD, the geometric means of ANCmax and ANC AUC0-last values were compared in ADA-positive and ADA-negative participants based on treatment with the pegfilgrastim-cbqv prefilled AI or the PFS (Supplementary Table S6). There was no impact of immunogenicity on PD based on treatment with the pegfilgrastim-cbqv prefilled AI or the PFS.
Impact of Immunogenicity on Safety
In both groups, TEAEs were similar in the ADA-positive and ADA-negative participants (Supplementary Table S7). There was no impact of immunogenicity on safety.
Discussion
The current study was designed to assess bioequivalence of pegfilgrastim-cbqv administered via prefilled AI and by PFS using PK and PD end points. The 90% CIs of the GMRs of the PK and PD parameters were within the required range (80–125%), demonstrating bioequivalence between the treatment groups. Bioequivalence was supported by the sensitivity analysis, which established the robustness of the primary PK bioequivalence results. Furthermore, the GMRs for the PK and PD parameters measured in this study are similar with those reported in a previous study that established PK and PD bioequivalence between pegfilgrastim-cbqv administered via PFS and pegfilgrastim (PFS) in healthy participants [13].
Pegfilgrastim-cbqv plasma levels were similar over time for both administration methods. ANC fluctuations were observed after pegfilgrastim-cbqv administration for both the prefilled AI and the PFS treatment groups. This ANC fluctuation aligns with the pegfilgrastim-cbqv mechanism of action, which causes a transient decrease in ANC secondary to the margination of peripheral neutrophils. This decrease is followed by a rapid and substantial increase in ANC due to demargination of peripheral neutrophils and the accelerated release of mature neutrophils from bone marrow [23].
The safety findings were similar between the pegfilgrastim-cbqv PFS and the prefilled AI. Most TEAEs were mild to moderate and were musculoskeletal effects (which are part of the known safety profile of pegfilgrastim-cbqv). The incidence of the common TEAEs was comparable between the treatment groups. Overall, injection site pain was the most common TEAE related to an ISR. The incidence of injection site pain was similar between the two groups. There were no new safety findings [2].
The incidence of ADAs was similar in both treatment groups. Titers of ADA-positive participants were generally low and against the PEG portion of pegfilgrastim-cbqv. Although NAbs were reported at baseline in three participants, no NAbs were reported in post-dose samples. There was no impact of immunogenicity on PK, PD, or safety.
Having multiple product presentations with different delivery systems is vital to ensuring the selection of the most suitable option that fits the user needs and preferences [10, 17, 24, 25]. AIs have been used for decades across a range of therapeutic areas [26, 27]. It is expected that the use of an AI to administer pegfilgrastim-cbqv will be beneficial to patients and health care professionals to provide options for self-administration and in-office use. Although patient self-administration was not evaluated in the current study, prior studies comparing the use of an AI and a PFS have shown an increased ease of administration and improved patient adherence [17, 24]. A reduced number of dosing errors in favor of the AI were also previously reported, by means of fixed dosing and consistent needle penetration depth [15, 17]. In addition, self-administration with an AI is preferred over the use of a PFS [17].
Self-administration of pegfilgrastim-cbqv using the prefilled AI the day after chemotherapy reduces the need for an additional clinic visit and, subsequently, minimizes exposure to unnecessary nosocomial infections in an at-risk patient group [10, 15, 17, 24]. Reducing the number of visits to a health care facility may particularly benefit patients living in geographically remote areas where significant travel is required for visits. Moreover, an AI may be seen as a more discreet administration method than an on-body injector; an AI also provides patients with a quick method of administration (< 10 s) and greater autonomy over dosing schedules, while decreasing anxiety associated with needle phobia [15, 16]. Furthermore, in the event of a nuclear or radiologic disaster resulting in many individuals with H-ARS, the pegfilgrastim-cbqv AI would provide users flexibility in administration. Overall, the availability of an AI provides an additional method of administration to choose from, which will likely be highly beneficial to certain patients and health care professionals.
Inclusion of only healthy male participants could be viewed as a potential limitation of the study. However, given the study objective, this population was justified: the purpose of the study was to compare two drug delivery systems rather than assess the drug overall, and healthy participants are recognized by the FDA as the most sensitive population for detecting PK/PD differences [28]. Additionally, previous data indicate there is no difference in the PK/PD of pegfilgrastim in males and females [2, 3, 13]. Safety data from real-world use of pegfilgrastim-cbqv administered via the prefilled AI will be collected as part of routine pharmacovigilance activities.
Conclusions
The results of the study showed bioequivalence by PK and PD of pegfilgrastim-cbqv administered using a prefilled AI compared with a PFS. The safety and immunogenicity profiles of pegfilgrastim-cbqv administered with the prefilled AI and the PFS were similar, with no new safety findings. Administration via a prefilled AI provides an alternative method of administration that could have several benefits compared with administration using the PFS.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27(suppl 5):v111–8.
UDENYCA (pegfilgrastim-cbqv) injection, for subcutaneous use. Package insert. Coherus BioSciences Inc.; 2022.
Neulasta (pegfilgrastim) injection single-dose prefilled syringe. Package Insert. Amgen, Inc.; 2021.
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hematopoietic growth factors Version 2.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed April 23, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. NCCN, National Comprehensive Cancer Network® (NCCN®)
Harrold J, Gisleskog PO, Perez-Ruixo JJ, et al. Prediction of survival benefit of filgrastim in adult and pediatric patients with acute radiation syndrome. Clin Transl Sci. 2020;13(4):807–17.
Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine. 2015;71(1):22–37.
López M, Martín M. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother. 2011;16(4):138–46.
Singh VK, Seed TM. Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems. Expert Opin Pharmacother. 2020;21(3):317–37.
Humphreys SZ, Geller RB, Walden P. Pegfilgrastim biosimilars in US supportive oncology: a narrative review of administration options and economic considerations to maximize patient benefit. Oncol Ther. 2022;10(2):351–6.
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404.
Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3.
Finck B, Tang H, Civoli F, Hodge J, O’Kelly H, Vexler V. Pharmacokinetic and pharmacodynamic equivalence of pegfilgrastim-cbqv and pegfilgrastim in healthy subjects. Adv Ther. 2020;37(10):4291–307.
FDA approves UDENYCA® autoinjector. Press release. Redwood City, CA: Coherus BioSciences, Inc.; 2023.
Roy A, Geetha RV, Magesh A, Vijayaraghavan R, Ravichandran V. Autoinjector - A smart device for emergency cum personal therapy. Saudi Pharm J. 2021;29(10):1205–15.
Hibino T, Yoshida T, Sagawa A, Masuda I, Fukuda T. Qualitative survey-based evaluation of operability and convenience for the etanercept biosimilar YLB113 in a unique injection pen in patients with rheumatoid arthritis. GaBiJournal. 2020;9(3):100–7.
Vermeire S, D’Heygere F, Nakad A, et al. Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence. 2018;12:1193–202.
Rekaya N, Vicik SM, Hulesch BT, McDonald LL. Enhancement of an auto-injector device for self-administration of etanercept in patients with rheumatoid arthritis confers emotional and functional benefits. Rheumatol Ther. 2020;7(3):537–52.
Borrás-Blasco J, Gracia-Pérez A, Rosique-Robles JD, Casterá MD, Abad FJ. Acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther. 2010;10(3):301–7.
Ypsomed Group. YpsoMate – the 2-step autoinjector. March 31, 2022. https://yds.ypsomed.com/en/injection-systems/auto-injectors/ypsomate.html. Accessed 24 May 2023.
Civoli F, Kasinath A, Cai XY, et al. Recommendations for the development and validation of immunogenicity assays in support of biosimilar programs. AAPS J. 2019;22(1):7.
Civoli F, Finck B, Tang H, Hodge J, O’Kelly H, Vexler V. Biosimilar pegfilgrastim-cbqv demonstrated similar immunogenicity to pegfilgrastim in healthy subjects across three randomized clinical studies. Adv Ther. 2022;39(3):1230–46.
Yang BB, Kido A. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 2011;50(5):295–306.
Schwarzenbach F, Dao Trong M, Grange L, et al. Results of a human factors experiment of the usability and patient acceptance of a new autoinjector in patients with rheumatoid arthritis. Patient Prefer Adherence. 2014;8:199–209.
Metz M, Semsek D, Rogmans G, Hutschenreuter U, Fietz T, Harde J, et al. Patient, nurse and physician preferences: final results of the CONVENIENCE study evaluating pegfilgrastim prophylaxis via pre-filled syringe or on-body injector (OBI) in cancer patients. Ann Oncol. 2020;31(supp 4):S1071 (Abstract 1884P).
The Business Research Company. Autoinjectors global market report 2023—by type (disposable autoinjectors, reusable autoinjectors), by therapy (rheumatoid arthritis, multiple sclerosis, diabetes, anaphylaxis, other therapies), by route of administration (subcutaneous, intramuscular), by distribution channel (online retailer, pharmacy), by end user (home care settings, hospitals and clinics, other end-users)—market size, trends, and global forecast 2023–2032. January 2023. https://www.thebusinessresearchcompany.com/report/autoinjectors-global-market-report. Accessed 24 May 2023.
Autoinjectors market size to hit around USD 247.3 BN by 2030. Press release. Globe Newswire; November 22, 2022. https://www.globenewswire.com/en/news-release/2022/11/22/2560680/0/en/Autoinjectors-Market-Size-to-Hit-Around-USD-247-3-BN-by-2030.html. Accessed 24 May 2023.
US Food and Drug Administration. Guidance for Industry. Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product. 2016. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM397017.pdf. Accessed 24 May 2023.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Chantell Hayward, PharmD, Cindi A. Hoover, PhD, and Chantel Kowalchuk, PhD, of Apothecom, San Francisco, CA. Support for this assistance was funded by Coherus BioSciences, Redwood City, CA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
Funding for this study was provided by Coherus BioSciences, Redwood City, CA. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Journal Rapid Service and Open Access Fees were also funded by Coherus BioSciences, Redwood City, CA.
Author information
Authors and Affiliations
Contributions
Hong Tang, Francesca Civoli, and Suzanna Tatarewicz contributed to the curation, formal analysis and validation of the data. Barbara Finck assisted with the formal analysis of the data. Francesca Civoli and Suzanna Tatarewicz contributed to project administration. Hong Tang conceptualized the study. All authors contributed to the study design of the manuscript, reviewed and approved the final content of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Hong Tang, Suzanna Tatarewicz, Nathalie Vandenkoornhuyse, and Barbara Finck are employees of and own stock in Coherus BioSciences (Redwood City, CA). Francesca Civoli is a former employee and owns stock in Coherus BioSciences (Redwood City, CA). Francesca is currently affiliated with Francesca Civoli Consulting LLC.
Ethical Approval
The Advarra Institutional Review Board reviewed and approved the protocol and informed consent form (IRB Registration Number: IRB0000971). All applicable laws and regulations were followed when conducting the study, including the International Conference for Harmonisation E6 Guideline on Good Clinical Practice. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tang, H., Civoli, F., Tatarewicz, S. et al. Pharmacokinetic and Pharmacodynamic Bioequivalence of Pegfilgrastim-cbqv Delivered via a Prefilled Autoinjector and Prefilled Syringe in Healthy Male Participants. Adv Ther 40, 4889–4906 (2023). https://doi.org/10.1007/s12325-023-02636-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02636-5