Abstract
Fungal keratitis, an ocular fungal infection, is one of the leading causes of monocular blindness. Natamycin has long been considered the mainstay drug used for treating fungal keratitis and is the only US Food and Drug Administration (USFDA)-approved drug, commercially available as a topical 5% w/v suspension. Furthermore, ocular fungal infection treatment takes a few weeks to months to recover, and the available marketed antifungal suspensions are associated with poor residence time, limited bioavailability (< 5%) and high dosing frequency as well as minor irritation and discomfort. Despite these challenges, natamycin is still the preferred drug choice for treating fungal keratitis, as it has fewer side effects and less ocular toxicity and is more effective against Fusarium species than other antifungal agents. Several novel therapeutic approaches for the topical delivery of natamycin have been reported to overcome the challenges posed by the conventional dosage forms and to improve ocular bioavailability for the efficient management of fungal keratitis. Current progress in the delivery systems uses approaches aimed at improving the corneal residence time, bioavailability and antifungal potency, thereby reducing the dose and dosing frequency of natamycin. In this review, we discuss the various strategies explored to overcome the challenges present in ocular drug delivery of natamycin and improve its bioavailability for ocular therapeutics.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Fungal keratitis is a one of the leading causes of monocular blindness and is associated with the poor management of the disease. |
This article offers the compressive review of modern approaches for the topical delivery of natamycin for efficient management of fungal keratitis. |
Nanotechnology-based modern approaches offer improved bioavailability and efficacy at reduced dose and dosing frequency. |
Much research has been done on natamycin delivery; however, no alternative dosage forms have reached the clinical stage, and the safety aspects need to be addressed. |
Introduction
Fungal or mycotic keratitis (FK) is a severe fungal ocular infection that infects the clear transparent corneal surface as well as the immediately associated layers (stroma, Descemet’s membrane and finally the anterior chamber). Acute fungal keratitis usually causes blurred vision owing to infection and corneal inflammation. The acuity can improve with treatment, but corneal scarring generally leaves the patient with worse acuity than before [1]. Recent investigations have reported that there are nearly 1.5 million new cases of fungal keratitis every year, and it is one of the corneal diseases that are leading causes of monocular blindness [2]. Fungal keratitis cases are most prevalent in the subtropical and tropical regions (20–60%) and developing countries, which may be largely related to the climate (e.g., high temperatures and relative humidity) and to any ocular trauma related to agriculture-related activities [3]. Several cases of fungal keratitis have been associated with ocular steroid [topical corticosteroids (dexamethasone)] treatment, recent surgery, ocular trauma, occupational ocular injury, using contact lenses and diseases like diabetes mellitus and HIV [4,5,6]. The causative organisms are species (sp) of fungi such as yeasts (Candida), filamentous fungi with septae (Fusarium, Aspergillus, Curvularia, Cladosporium) and filamentous fungi without septae (Rhizopus) [6]. About 24–30% and 37–62% cases of FK are caused by Aspergillus and Fusarium sp., respectively. Due to the fast-advancing nature of Fusarium-related keratitis, the cases are more severe and less responsive to drug therapy than those of Aspergillus keratitis [6, 7].
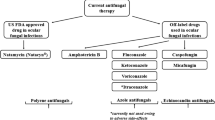
Currently, three classes of antifungal agents are used to treat FK: triazoles, polyenes and echinocandins [8]. Natamycin (Nat) is a natural tetraene antibiotic also known as pimaricin, which belongs to a large class of polyene antifungals obtained by the fermentation of Streptomyces natalensis (gram-positive bacterium) [9]. Due to its broad spectrum of fungistatic activity and strong antifungal potency and enhanced corneal penetration, it is regarded as the front-line drug in the treatment of FK [10,11,12]. It was first isolated from fermented broth from a South African soil sample of Streptomyces natalensis by Gist-brocades Research Laboratories (The Netherlands) in 1955. Streptomyces natalensis and Streptomyces gilvosporeus are used for fermentation for industrial production [13, 14].
Nat is a BCS class II with an aqueous solubility of 30–50 mg/l at physiologic pH and is given as a suspension. It is more readily soluble in highly acidic and alkaline solutions but undergoes faster degradation under highly acidic and alkaline conditions [15]. It is a high-molecular-weight molecule (665.733 g/mol) with limited corneal permeation. It is prone to photo-oxidation and photodegradation upon exposure to heat, light, oxidants, heavy metals, extreme pH values and temperatures > 100 °C [16] attributed to chromophore cleavage caused by light absorption [17].
Despite the difficulties in using Nat to treat ocular fungal infections, it is still recommended in clinical practice for the superficial treatment of ophthalmic fungal infections. This may be attributed to the clinical efficacy or safety profile of Nat eye drops in fungal infections, where Nat showed fewer side effects, lower resistance rates and lower ocular toxicity reactions compared to antifungals, such as amphotericin B and azoles [11, 18, 19]. To better understand the recent advancement in Nat ocular delivery, this review summarizes the various nanoformulations developed and studied with an aim to improve the corneal penetration and bioavailability of Nat, thereby reducing its dose and dose frequency. Furthermore, few reports of comparative studies conducted on amphotericin B, voriconazole and Nat are discussed, which highlight the superior efficacy of Nat in treating FK infection as well as the currently available marketed Nat suspension, and the challenges faced are briefly described. Finally, a brief description of Nat patents and future prospects are presented.
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Methods
A search was conducted on PubMed, Scopus and ScienceDirect using relevant keywords such as “natamycin,” “fungal keratitis,” “natamycin + ocular delivery” and “novel delivery system” and the marketed formulations of natamycin. The search identified various clinical trials and comparative case studies investigating the action of natamycin and various antifungal agents in the treatment of fungal keratitis, especially in the treatment of Fusarium keratitis cases, which are the most severe and least responsive cases of fungal keratitis. Furthermore, we examined an array of articles highlighting the various drawbacks of the currently commercially available natamycin 5% w/v suspension and current research studies on novel drug delivery strategies conducted to overcome the challenges and overall dose reduction and dosing frequency, consequently reducing the adverse effects associated with natamycin suspension. We extensively reviewed the literature and summarized it in this review article.
Comparative Studies of Nat Against Other Antifungal Drugs
Despite their potential antifungal activity, other antifungal medications, such as amphotericin B, flucytosine and fluconazole, have been reported to cause systemic toxicity [19]. Existing antifungal agents tend to fail to restore vision to its initial level after ocular infection [20]. Several comparative studies have been carried out with Nat, amphotericin B, voriconazole and other antifungal drugs against fungal species such as Fusarium and Aspergillus isolated from corneal infections to investigate which antifungal has the superior efficacy in treating a specific type of FK infection [21].
Prajna et al. compared the Mycotic Ulcer Topical Treatment Trial I (MUTT I) clinical outcomes of topical Nat and topical voriconazole therapy for filamentous fungal keratitis and found that Nat treatment showed significantly improved clinical and microbiologic outcome especially against Fusarium sp. and that perforation of the cornea and/or therapeutical corneal transplant was more prevalent in voriconazole-treated than Nat-treated individuals [11]. Similar results were observed when Sharma et al. compared the efficacy of topical 1% voriconazole and 5% Nat in treating fungal keratitis. Nat-treated cases showed greater improvement in vision especially in patients with Fusarium keratitis compared to those treated with voriconazole [22].
A similar study was reported by Spierer et al. comparing the in vitro antifungal activity of Nat (5%), amphotericin B (0.15%), fluconazole (0.2%) and voriconazole (1%) against Candida spp. isolates from human corneas. The results demonstrated that 100% of the samples showed growth inhibition upon treatment with Nat and amphotericin B compared to 19.6% and 85% overall inhibition rates of samples by fluconazole and voriconazole, respectively. For Candida keratitis, Nat and amphotericin B are the drugs of choice as voriconazole requires a higher concentration to inhibit Candida for the treatment of Candida keratitis [12].
Several researchers have investigated the minimum inhibitory concentrations (MICs) of different antifungals against different FKs causing fungal species (sp.). Cordeiro et al. investigated the antifungal potency of amphotericin B and observed that higher concentrations of amphotericin B (0.0625–4 μg/ml) were required to inhibit Candida sp. and about 19 species of Candida were resistant to it (MIC > 1 μg/ml) [23]. Similarly, a study by Pfaller et al. on invasive candidiasis patients in phase III clinical trials suggested that most Candida sp. growth can be inhibited by 0.25–0.5 μg/ml voriconazole; however, few isolates were resistant and required > 1 μg/ml voriconazole [24]. Nat has demonstrated variable MIC against Candida ranging from 1–2 μg/ml for C. albicans [25, 26] and may be as high as 150 μg/ml for C. parasilopsis as reported in the study by Salvosa et al. [27].
An in vitro antifungal study by Cόrdoba et al. against 29 Fusarium isolates suggested that MIC of amphotericin B and voriconazole ranges from 0.5 to > 16 μg/ml and 2–16 μg/ml, respectively [28], while that of Nat was found to be 4–8 μg/ml against 20 strains of Fusarium sp. [29]. A similar study carried out by Arikan et al. against 82 Aspergillus sp. found amphotericin B and voriconazole MICs to be 0.5 to 4 μg/ml and 0.125–2 μg/ml, respectively [30], whereas Nat exhibited an MIC range of 5–40 μg/ml against 191 sp. of Aspergillus [31]. Based on different studies, it can be concluded that voriconazole exhibits a narrower range of MIC with no remarkable difference among the three different fungal sp. and showed the highest MIC range for the Fusarium sp. Nat exhibits a higher and variable MIC range for the different fungal species but has excellent efficacy against Fusarium, which is the major causative fungus of FK. Table 1 summarizes the MIC of Nat, amphotericin B and voriconazole against three different fungal species.
Current Marketed Products and Their Challenges
Nat is the only topically administered ocular antifungal drug approved by the US FDA and is commercially available as a 5% w/v ophthalmic suspension [20, 32, 33]. Some of the commercially available Nat eye drops are provided in Table 2. According to a global survey, 80% of ophthalmologists treating FK propose that current medications are only moderately efficient in treating corneal fungal infections [34]. Reports suggest that fungal infections can be resolved with topical therapy in only 7.6% of patients, while 92.4% require surgical intervention [35]. Another disadvantage of existing topical treatments is that they must be administered numerous times a day over a long period, increasing the patient’s treatment expense [7]. Nat suspension therapy is associated with poor corneal retention attributed to drug dilution and increased tear fluid turnover or due to nasolacrimal drainage and non-specific absorption [36, 37] with only about < 5% of the drug reaching the intended ocular tissue. A longer duration of therapy (4–6 weeks) is required to achieve optimal drug concentrations and to treat the infected ocular site, which is achieved by a high dosing frequency (1 drop every 1–2 h for first 3–4 days followed by 6–8 times daily for 14–21 days) [38]. The higher dosing frequency may cause patient non-compliance or even hospitalization [39,40,41]. The ocular anatomical layers restrict drug penetration leading to poor absorption, and the high tissue binding potential of Nat leads to low bioavailability (< 5%) [42, 43] and is not recommended for treating deep stromal fungal infections. The long dosing schedule of Nat suspension with shorter residence time at the site of action causes failure of therapy and an increase in resistance to FK [32, 44]. The eye suspension is reported to cause several adverse effects such as irritation, discomfort and eye pain, allergic reactions (not significant), chest pain, eye hyperemia and edema, corneal opacity, changes in vision, dyspnea, paresthesia, foreign particle sensation and tearing caused by longer duration of therapy [38].
Novel Formulation Strategies for Nat Ocular Delivery
Despite its widespread use, formulating Nat formulations that provide both targeted and efficient antifungal action is arduous [9]. Owing to the presence of a complicated anatomy and many static and dynamic barriers, achieving an effective drug concentration in the eye via the topical route is complicated. Low ocular bioavailability (< 5%) is attributed to the rapid clearance from the precorneal region and minimal corneal permeability of conventional drug formulations. Therefore, the development of a drug delivery system with improved precorneal retention and sustained drug release over long periods would be a potential strategy for therapeutic interventions for ocular surface infections [41].
The administration of conventional eye drops is convenient but leads to suboptimal management of the disease. Novel topical drug delivery techniques can enable better corneal permeability and ocular retention, thereby maintaining the Nat MIC in the lacrimal fluid, which helps to treat the infection with minimal to no risk of development of resistance and recurrence [45]. With the advancement of nanomedicine and nanotechnology, various novel therapeutic approaches and strategies for delivering drugs to their intended targets have been proposed [46]. Several researchers around the globe have explored novel approaches to Nat ocular administration such as nanoparticles, niosomes, nanomicelles, cell-penetrating peptides, nanocrystals, nanofibers, etc., to improve the ocular bioavailability and efficacy of Nat against FK. In this review we have briefly reviewed a few studies done by some researchers and Table 3 summarizes the advantages, drawbacks and stage of experimentation of the various novel natamycin delivery systems (Fig. 1).
Colloidal Nanocarriers
Polymeric Nanoparticles
Nanoparticles (NPs) are mucoadhesive, biodegradable and composed of drug molecules dissolved or encapsulated in a matrix of natural or synthetic polymers (polycaprolactone, albumin, sodium alginate and chitosan). Nanoparticles are promising candidates because of their small size (50–400 nm), which results in reduced eye irritation and improved permeability owing to the longer residence time of the drug at the ocular surface. It provides sustained drug release by acting as a drug reservoir, leading to reduced dose and dosing frequency [47,48,49].
Chandasana et al. designed a Nat-encapsulated 1% and 5% poly-d-glucosamine (PDG) functionalized polycaprolactone (PCL) nanoparticle suspension for prolonged corneal targeted drug delivery. The 5% PGD-PCL Nat-loaded NPs showed significantly higher Cmax/MIC90 (~ tenfold) and AUC(0–10)/MIC (~ 125 fold) compared to Natamet®. Amino groups of PDG (cationic polysaccharide) have a higher binding affinity to anionic mucosal sialic acid residues in the corneal epithelium, which aids in improved precorneal retention at the ocular surface. Furthermore, PDG is reported to have fungicidal activity and ocular biocompatibility and is known to enhance the paracellular transport of drugs [50].
Polymeric NPs have the potential to sustain drug release and improve pharmacokinetics compared with conventional eye drops. Nat encapsulated lecithin/chitosan (LC/CS) NPs showed sustained drug release for up to 7 h compared to 2 h with Natamet®. The AUC(0–∞) of the drug increased by 1.47-fold, while the clearance of Nat decreased by 7.4-fold compared to the Natamet® suspension. CS is a cationic polyelectrolyte that has a strong affinity for the anionic corneal surface, resulting in an increased retention time. CS offers ophthalmic biocompatibility, non-toxicity, biodegradability and mucoadhesion [10] whereas LC can enhance the lipophilic drug loading and sustained drug release because of its lipophilic nature [51]. When choosing a treatment protocol and dosage regimen for an antifungal drug like NAT that exhibits concentration-dependent killing, the PK/PD indices are acknowledged as a vital element.
Recently, Sha et al. developed a thermosensitive Nat-loaded tri-block polymer NP-poloxamer gel complex and studied its safety, efficacy, penetrability, adhesion and antifungal activity in the treatment of FK. The tri-block polymer consisted of poly-lactic-co-glycolic acid, polyethylene glycol and poly-ethylene imine, which provided greater penetrability, stability and utilization of the loaded drug. The micro-double dilution method was used to evaluate the antifungal efficacy of Nat-loaded tri-block polymer NPs, and they were compared with 5% marketed Nat suspension. The study showed that the MIC of the Nat NPs was 2 μg/ml, which was half the MIC of the Nat solution (4 μg/ml). The cytotoxicity test showed that the Nat NPs had a higher cytotoxicity to human corneal epithelial (HCE-T) cells than the marketed suspension, which might be attributed to the cytotoxicity or inhibition of cell proliferation (rather than killing of cells) by the polymer block, and the cytotoxicity of both formulations was concentration dependent. The in vivo trans-corneal penetration study, using male New Zealand white rabbits, showed that 2.5 mg/ml Nat NP-thermosensitive gel formulation had a therapeutic effect similar to 50 mg/ml Nat suspension and showed sustained release, with improved penetrability and higher residence time, which effectively increased Nat content in the eye without causing epithelial injury and inflammatory cell infiltration [52].
Lipid Nanoparticles (NLC and SLN)
Lipidic nanoparticles are spherical, submicron, lipidic organic nanocarriers (50–1000 nm) with a biodegradable and biocompatible solid lipid or a mixture of solid and liquid lipid cores, which entrap lipophilic or hydrophobic drugs, and an exterior surfactant shell that may be sustained in the ocular tissue system [49].
Solid lipid nanoparticles (SLNs) constitute a drug that is encapsulated in a solid lipid matrix. SLNs are reported to be the most effective colloidal drug delivery systems compared to liposomes and polymeric nanoparticles because of their non-toxicity, protection of the drug from degradation, better stability, enhanced drug bioavailability and sustained drug delivery [53, 54] with lower production costs. Furthermore, SLNs have desirable characteristics, such as biocompatibility, larger surface area, prolonged drug release, reduced clearance, improved penetration across ocular barriers and chemical preservation of unstable drug molecules with higher drug inclusion properties [48, 55, 56].
Khames et al. prepared Nat SLN formulations that were optimized using Box-Behnken statistical design (three-level, three-factor). The optimized Nat SLN formulation showed an initial burst release within the first 2 h followed by a sustained drug release for 8 h when studied using the modified rotating paddle dialysis bag diffusion technique. The initial burst release might be attributed to the surface adsorption of Nat and solubilization of the outer layer of the particle. Furthermore, Nat SLN significantly improved antifungal activity against Aspergillus fumigatus and Candida albicans, as the minimum inhibitory concentration against the fungal strains was lowered by 2.5-fold compared to the unformulated Nat when tested using modified Kirby-Bauer method and broth micro-dilution method [57]. The (Fig. 2) gives the histopathologic images of the corneal irritation studies conducted on the goat corneas.
Histopathologic images of corneal irritation studies performed on freshly excised goat corneas using the optimized NAT-SLN formula. The negative control and optimized NAT-SLN formulation showed no signs of morphologic damage to the epidermal corneal layers and no ocular irritation on the corneal surface structure compared to 0.1% SDS (sodium dodecyl sulfate), which showed significant damage to the epidermal layer of the cornea. Reproduced from [57] under Creative Commons Attribution License
Abdelmonem et al. formulated Nat SLNs (using lipids + Tween 80 or Pluronic F127) loaded in a mucoadhesive gel using Carbapol 940 and hydroxypropyl methylcellulose (HPMC) using a 23 factorial design, which showed sustained in vitro release of Nat from the gels for 6 h (dependent on polymer concentration) compared to the marketed product. An in vitro antifungal study was performed using agar cup diffusion method against C. albicans, which reported that Nat-SLN with 5% Pluronic F127 and 1:1 mixed lipid-loaded 4% HPMC gel had the maximum zone of inhibition compared to the other formulations and the marketed product and hence was further analyzed for in vivo antifungal study on rabbits. The histopathologic and clinical findings confirmed that the above formulation provided maximum curing efficacy against Candida-related FK compared to the marketed product with a controlled release of up to 8 h, which in turn reduced the dosing frequency, thereby improving patient compliance [58].
Nanostructured lipid carriers (NLCs) are second-generation SLNs that exist as solid lipid matrices at body or room temperature and are structurally composed of a unique mixture of solid (fat) and liquid (oil) lipids with a formless, uneven (less ordered) lattice arrangement [59, 60]. NLCs exhibit enhanced pre-ocular residence time, which improves penetration and bioavailability as well as stability on storage with low toxicity [61, 62]; in addition, they address the major disadvantages associated with SLN, such as poor drug loading and drug expulsion during storage [48].
Patil et al. were the first to fabricate Nat-loaded PEGylated NLCs using a Box-Behnken design and evaluate them against a marketed formulation. The transcorneal penetration (across isolated rabbit corneas) of 0.3% Nat-loaded PEGylated NLCs was ~ twofold higher than that of Nat-NLCs and ~ sevenfold higher than that of Natacyn®. An in vivo distribution study showed that PEGylated NLCs had a ~ twofold higher Nat concentration in the cornea and iris-ciliary body (ICB) compared to diluted Natacyn® (0.3%), suggesting improved corneal permeation of NLCs compared to Nat suspension [63].
Vesicular Systems
Niosomes
Niosomes are novel vesicular carrier systems (10–100 nm) [64] with a closed bilayer structure formed by the self-assembly of a non-ionic surfactant, where the central aqueous core entraps the hydrophilic drugs, while the lipophilic drug partitions between the bilayer hydrophobic tails [65]. Niosomes offer several advantages over liposomes, such as higher permeation potential, improved bioavailability, better chemical stability, controlled release characteristics with delayed clearance, better biocompatibility and biodegradability, and non-immunogenicity, and they are reported to have fewer side effects than liposomes in vivo [66,67,68].
El-Nabarawi et al. developed a dual-purpose Nat-loaded niosomal gel containing (0.5%) ketorolac tromethamine (KT) to improve Nat penetration through the cornea and decrease the inflammation caused by FK. The in vitro drug release of niosomal formulations analyzed using a membrane diffusion technique showed an initial burst release within the first 3 h attributed to diffusion and desorption of Nat present on the niosomal surface followed by sustained release (77.49% after 24 h) compared to Natacyn®, which showed 85.52% drug release after 8 h. The optimized niosomal formulation was then incorporated into a gel containing 0.5% KT. The Nat niosome-loaded gel showed HPMC-E4 and sodium carboxymethylcellulose (NaCMC) concentration-dependent drug release. The Nat gel analyzed for in vitro release using dialysis method showed prolonged release for up to 24 h, which might be attributed to the highly viscous diffusion barriers formed by the hydrated polymer chains. The antifungal study was carried out using agar well diffusion technique against C. albicans, which demonstrated 8 μg/ml MIC for 4% Na.CMC niosomal gel compared to the 63 μg/ml MIC of 2% HPMC-E4; this may be attributed to the higher viscosity of the gel, which resulted in poor diffusion of Nat. The in vivo visual assessment showed no corneal irritation. Furthermore, histopathologic studies showed a significant and the highest reduction in the fungal load and inflammation of the cornea in the 4% Na.CMC niosomal gel-treated group compared to the 2% HPMC-E4 niosomal gel group. The combination (Nat-loaded niosomes/0.5% KT- 4% Na.CMC) enhanced the permeability through the cornea and ocular bioavailability, which reduced the dosing frequency, thereby reducing the systemic side effects, relieved the inflammation caused by Candida and improved patient compliance [65]. Similar results in ocular irritancy and ex vivo transcorneal permeation studies (Fig. 3) were obtained by Paradkar et al.
Ocular irritancy study images of rabbit eyes: a untreated left eye; b optimized niosomal in situ gel formulation-treated right eye after exposure for 1 week. No symptoms of ocular irritation, ocular damage or any abnormal clinical signs of the cornea, iris or conjunctiva were observed. Images of the ex vivo transcorneal permeation study carried out using niosomes labeled with rhodamine B (fluorescent dye) and observed under a confocal microscope; c the red dot indicates the internalization of niosomes into the cells across the cornea; d the corneal tissue surface-stained red with the dye showing bright red fluorescence. The niosomal formulation showed enhanced therapeutic effects owing to greater permeation across deeper layers of the cornea. Reproduced from [70] with permission from Elsevier
Verma et al. developed cationic mucoadhesive N-trimethylchitosan (TMC)-coated Nat-loaded niosomes. The TMC-coated Nat niosomes were reported to have a 1.5-fold higher AUC(0–12) and 27 times higher MRT than the marketed formulation. It was also observed that niosomes containing a 2% w/v dose of Nat showed optimum efficacy and reduced toxicity at the nasolacrimal site while providing a sustained release for 12 h, which resulted in reduced dose and dosing frequency. The Nat-containing formulations exhibited time- and concentration-dependent killing of C. albicans when evaluated using a time-kill kinetics assay, which is conducted to assess the speed at which killing occurs at a given drug concentration [69].
Glycerosomes
Glycerosomes are modified liposomes, which are colloidal concentric bilayer structures composed of phospholipids, water, high amounts of glycerol (10–30% v/v) and cholesterol [71]. Glycerosomes are preferred over conventional liposomes owing to their higher stability and fluidity. Glycerol enhances the deformability index of glycerosomes, providing better penetration across the ocular barriers. Glycerosomes have been explored for topical and skin drug delivery and are reported to be safe and non-toxic [71,72,73].
Gupta et al. developed novel Nat glycerosome eye drops and studied their potential antifungal activity compared with conventional Nat liposomes and marketed Nat eye drops. Glycerosomes showed increased entrapment efficiency (80.84%) compared to conventional liposomes (59.5%). Furthermore, the Nat glycerosomes showed enhanced in vitro drug penetration (93.422%) and provided a controlled and better retention effect near the cornea compared to the conventional liposomes (57.6%) and Nat eye drop when performed using the Franz diffusion cell method with a dialysis membrane. The ex vivo studies performed on excised goat corneas exhibited relatively higher corneal penetration by the glycerosomes (~ 80%) compared to the conventional liposomes (~ 75%) and the Nat eye drops (~ 20%). The superiority of glycerosomes over conventional liposomes was attributed to the large glycerol vesicles, which have a higher drug entrapping efficacy [74].
Bilosomes
Bilosomes are novel, closed, stabilized vesicles composed of nonionic amphiphilic surfactants in a bilayer that contain bile salt nanovesicular carriers and resemble niosomes [75, 76]. Bilosomes are highly flexible and ultra-deformable vesicles that help in drug penetration across the cornea, provide higher drug stability and have been extensively studied for improving drug delivery through oral and transdermal routes. Bilosomes offer a potential alternative to ocular drug delivery because of the presence of surfactants and bile salts that protect the drug against enzymatic degradation and also provide stability and higher permeation across ocular barriers owing to the nanosized vesicles [75, 77].
Janga et al. investigated Nat bilosome-loaded ion-sensitive in situ gels by using gellan gum and xanthum gum. Gellan gum in situ gel showed superior mucoadhesion and rapid sol-gel conversion compared to xanthum gum. In vitro cytotoxicity evaluated by methyl thiazolyl tetrazolium (MTT) assay and histology studies performed on rabbit corneas (Fig. 4) demonstrated the non-toxicity of gellan gum gel to corneal epithelial cells. The ex vivo study conducted on excised rabbit eyes suggested improved transcorneal flux by six- to ninefold compared to Natacyn®. The ocular disposition studies suggested significantly higher Nat concentration in ocular tissues in the biolosome-loaded in situ hydrogel-treated group compared to Nat suspension after 6 h. Gellan gum and xanthum gum are anionic polysaccharide polymers specifically used in the study for their ion-sensitive gelling property. The formed hydrogels help in improving the pre-corneal residence time, and the shear thinning pseudoplastic behavior is important for eye comfort when blinking [78].
Histologic images (10 × and 100 × magnification, respectively) of rabbit corneas exposed to A DPBS (2.5% w/v randomly methylated-β-cyclodextrin), B control, C natamycin bilosomes (NB 2) and D natamycin bilosome in situ gel (NBG 2) formulations. There were no signs of damage to the corneal structure in the rabbit eyes treated with NB 2 and NBG 2, indicating corneal compatibility and safety, and the corneas were structurally similar to those treated with a control marketed suspension and DPBS. Reproduced from [78] under Creative Commons Attribution License
Transferosomes
Transferosomes are ultra-deformable modified liposomes prepared using an edge activator and a hydrophilic/amphiphilic single-chain surfactant, which impart superior transmembrane permeability. The edge activator regulates the vesicles according to the corneal epithelial pores aiding in smooth permeation through the anatomical ocular barrier. The external stress causes the transferosome vesicles to change shape quickly, attributed to the edge activators, which diminishes the interfacial tension, increasing the carrier system's propensity to shrink [79,80,81,82,83].
Janga et al. developed Nat-loaded transferosomes incorporating ion-sensitive in situ gel using gellan gum. The corneal histologic studies have demonstrated biocompatibility, suggesting that transferosomes are a safer approach for ocular delivery. Nat-loaded transferosomes and in situ gel showed ~ 9- and ~ 16-fold improved corneal permeability compared to a drug suspension, respectively. The improved corneal permeation of in situ gel across the excised rabbit corneas (with a small portion of sclera) might be attributed to higher hydrophobic interactions or hydrogen or ionic bonding between gellan gum and cellular glycoproteins of gellan gum, imparting greater mucoadhesion [84].
Cubosomes
Cubosomes are unique bicontinuous cubic nanovesicles prepared using liquid-crystalline cubic clusters with high surface areas and microstructures. Cubosomes have the capacity to self-assemble in water to form 100–500-nm size range cubosomes with a structure comparable to that a of honeycomb with internal aqueous channels [85, 86]. Cubosomes have the potential to encapsulate hydrophilic, hydrophobic and amphiphilic drug molecules with additional bioadhesive properties, sustained release effect and easier biodegradability by the enzymes, which enhances drug residence time at the corneal surface along with a reduction in dosing frequency [87, 88].
Hosny et al. investigated Nat cubosomes loaded with a pH-sensitive in situ gel using 1.5% Carbopol 934 to reduce the dose and dosing frequency and overcome the side effects associated with marketed eye drops. The in situ gel improved precorneal retention and showed a 3.3-fold enhancement in ex vivo Nat permeation (performed on excised corneas of albino rabbits) compared to the 2% Nat marketed suspension and 5.2 fold compared to the Nat suspension. The in vivo ocular irritation study conducted on New Zealand white rabbits suggested that the developed cubosomal formulation was safer and less irritating compared to the marketed formulation [46].
Kazi et al. studied the use of Nat cubosome nanoparticles by implementing a 32 factorial design, aiming to improve corneal permeation. Cubosomes demonstrated an initial burst release of the drug, followed by sustained drug release for up to 8 h. The ex vivo corneal permeation studies performed on freshly excised goat corneas revealed higher apparent permeability (20.59 × 10–2 cm/h) and flux (5.46 mol/h) of the cubosomes, which reinforced improved Nat corneal permeation attributed to the nanosize and lipophilic structure of the cubosomes compared with the pure drug suspension. The Draize test demonstrated the non-irritant property of the cubosomes with a zero irritation score and no ocular damage [89].
Miscellaneous
Micelles
Micelles are self-assembled nanocarriers composed of a lipophilic core and hydrophilic shell composed of surfactants or amphiphilic copolymers. The lipophilic core aids in specifically entrapping lipophilic drugs, while the hydrophilic portion serves as a steric barrier that prevents aggregate formation and promotes solubility in aqueous or biologic media, such as lacrimal fluid. The micelles remain stable for a long time, resulting in delayed clearance, thereby providing tailored drug release by controlling their size, composition, functionality and overall morphology [90,91,92,93].
Guo et al. synthesized self-assembling mucoadhesive poly (ethylene glycol)-block-poly (glycidyl methacrylate) (PEG-b-PGMA) micelles to reduce the Nat dosing frequency for the treatment of fungal keratitis. The in vivo antifungal study concluded that the dosing frequency of Nat may be reduced from eight to three times/day owing to the sustained release of Nat from micelles with good clinical effects. The Nat micelles were non-irritant and biocompatible, which was proved by the results of MTT assay, and imparted improved Nat solubility and corneal permeability. This was attributed to the mucoadhesive nature of the PEGylated shell of the micelles, which increased their retention time in the mucosal layer of the tear film. An in vitro release study carried out using the dialysis method showed an initial burst release due to the release of the loosely (untrapped) trapped free Nat on the micellar structure followed by a plateau phase [94].
Lorenzo-Veiga et al. developed polymeric nanomicelles using Soluplus and Pluronic P103 dispersions, with or without α-cyclodextrin (10% w/v), to improve aqueous solubility and enhance ocular penetration. Nat solubility was enhanced by 6-, 3.27- and 2.77-fold with Soluplus, Pluronic P103 and their mixed micelles, respectively. Vertical Franz diffusion cells were used to study in vitro drug release, and the results showed that Pluronic P103 (10%) micelles had the fastest drug release in 6 h with faster diffusion and a higher permeability coefficient, which was attributed to the smaller size and less compact structure of the micelles compared to mixed micelles and Soluplus. The poly(pseudo)rotaxanes (ternary complexes) system increased the viscosity of the micellar formulation, thereby increasing the residence time at the corneal surface and also causing a decrease in drug diffusion compared with the corresponding micelles. The ex vivo study conducted on excised bovine eyes using vertical diffusion Franz cells demonstrated that Soluplus-based poly(pseudo)rotaxanes improved the solubility of Nat in the aqueous layer surrounding the cornea leading to an increase in drug accumulation but showed the lowest permeability coefficient, attributed to the larger size of the encapsulating carrier and hindrance by the ocular tissue. The HET-CAM assay suggested that all the micellar formulations were non-irritant [95].
Cell-Penetrating Peptide (CPP)
CPPs, also known as protein translocation domains (PTD), membrane translocation sequences or Trojan horse peptides, are a family of peptides with generally 5–40 amino acid (aa) sequences. CPPs can traverse human, plant and bacterial cell tissues and membranes via energy-dependent or -independent pathways, with no interaction with receptors. CPPs possess antimicrobial properties with low cytotoxicity and facilitate the cellular internalization of covalently or non-covalently bound drug molecule [96]. Since the discovery of TAT peptide as a cell-penetrating peptide in 1988, a slew of CPPs has been discovered, produced and studied for use in the treatment of many disorders [97]. Johnson and coworkers' peptide for ocular delivery (POD) was the first CPP formulated for ocular administration in 2008 [98]. KAI9803, RT001, RT002 and XG-102 are some of the CPP-conjugated therapeutics currently under clinical studies for the management of ocular diseases [96, 99].
Jain et al. was the first to conjugate Nat with Tat-dimer (Tat2 and MTat2), a CPP, to elucidate Nat uptake ability in corneal epithelial cells and antifungal activity. The study revealed an increase in Nat solubility of up to 100-fold in water and showed enhanced cellular penetration potential. A small concentration of Nat-conjugated CPPs (10 μM) when assessed against Fusarium solani using a two-fold microbroth dilution assay showed that it completely inhibited the growth of F. solani, thereby exhibiting a twofold enhancement in antifungal activity compared to unconjugated Nat [7].
Rohira et al. conjugated Nat with Tat2 and evaluated its tissue penetration potential and antifungal efficacy compared to the Nat (5% w/v) marketed formulation. The topically applied Nat-Tat2 showed a five-fold higher ocular penetration and accumulation in aqueous and vitreous humor, which indicates improved Nat bioavailability compared to pure Nat. The cytokine study of Nat-Tat2 using the Cytokine Bead Assay Kit showed a marked reduction in IL-6 while Tat2 reduced levels of IL-6 and IL-1β. For efficient antifungal activity against Fusarium sp. in vivo, the Nat-Tat2 conjugation significantly reduced the Nat dose from 5% w/v (marketed formulation) to 0.330 mg ml−1 (Nat-Tat2 concentration equivalent to 100 × 10–6 M) [100]. The (Fig. 5) gives the grade reduction and cumulative clinical scores pre-treatment and post-treatment using slit-lamp biomicroscopy performed on New Zealand white rabbits.
Effect of PBS, 5% natamycin suspension, Tat2natamycin and Tat2 on grade reduction and cumulative clinical scores pre-treatment (a–f) and post-treatment using slit-lamp biomicroscopy performed on New Zealand white rabbits. Representative images of grade reduction in each group after 6 days of treatment: a PBS (n = 7), b 5% natamycin suspension (n = 8), c Tat2natamycin (200 × 10−6 M) (n = 8), d Tat2natamycin (100 × 10−6 M) (n = 9), e Tat2 (200 × 10−6 M) (n = 7) and f Tat2 (100 × 10−6 M) (n = 7). After 6 days of treatment, the animals treated with 5% natamycin suspension showed accumulation of the drug around eyelids leading to discomfort and difficulty in opening the eye, and animals with lower grades of fungal keratitis (grade 0–2) showed increased body weight and physical activity. About 44% and 38% of animals exhibited complete resolution of the disease with no vascularization or endo-exudates after being exposed to Tat2natamycin (100 × 10−6 M) and (200 × 10−6 M), respectively, compared to 5% natamycin suspension, while the animals treated with Tat2 200 × 10−6 M showed higher vascularization with about 29% and 14% showing complete resolution upon treating with 100 × 10−6 M and 200 × 10−6 M of Tat2, respectively. Reproduced from [100] with permission from Elsevier
Nanocrystals
Nanocrystals are pure drug nanoparticles with sizes ranging from 1 to 1000 nm and have a higher drug-loading capacity than other nanocarriers, which aids in the efficient transport of drug molecules into cells. Nanocrystals provide higher saturation solubility, faster dissolution rate, higher mucoadhesion and enhanced saturation solubility, leading to a higher concentration gradient and resulting in a greater diffusive flux at the ocular surface. Furthermore, nanocrystals with 100% drug loading may result in longer retention at the ocular surface [101,102,103].
Das et al. synthesized Nat nanocrystal-loaded pH-sensitive in situ gels using Carbopol 940P and HPMC EL 50 V by implementing the Box-Behnken design and response surface curve to obtain optimized Nat nanocrystals that show improved solubility and absorption of Nat. The in vitro release study revealed faster drug release from nanocrystals (87.56% after 2 h) than from the pure drug. The in vitro diffusion of the in situ gel showed sustained drug release compared to the marketed formulation attributed to the retardant effect of the gel, which aided in improving the ocular bioavailability of the gel. The ex vivo studies demonstrated higher corneal permeation of the in situ gel attributed to extended dissolution compared to the marketed formulation [104].
Nanofibers
Nanofibers are nanosized biodegradable polymeric drug carriers with large surface areas obtained by electrospinning and sol-gel processes. They offer high drug-loading potential, increased drug encapsulation efficiency and co-administration ability. Fibers can also aid in drug transit across physiologic barriers, reducing premature drug release, targeted delivery and controlled drug release [105, 106].
Veras and Ritter et al. fabricated and characterized poly-ε-caprolactone (PCL) Nat nanofibers by electrospinning four formulations. Formulations with tetrahydrofuran (THF): N, N-dimethyl formamide (DMF) (4:1, v/v), polyethylene glycol (PEG 4000), and medium-chain triglycerides (MCT) dissolved in THF: chloroform (CHCl3) (3:1 v/v) showed higher Nat loading in the PCL nanofibers, resulting in enhanced antifungal activity against eight different yeast and filamentous fungal isolates. These nanofibers showed low hemolytic damage (1.2–6.5%) when hemolysis assay was conducted on heparinized sheep blood as a sufficient amount of Nat was not freely available to damage cells [107].
Contact Lenses (CL)
Contact lenses (CLs) are ocular prosthetic devices that can provide a combination of vision correction and ocular therapeutics, as reported by Sedlacek in 1965 [108]. The post-lens tear film is a fluid layer that separates the CLs from the cornea. It has a clearance time of approximately 30 min, which provides a prolonged contact time with the cornea compared to the few minutes of conventional eye drops [109, 110]. It has been reported that about 50% of the drug released from the CLs can diffuse into the cornea, which is 35-fold more efficient than eye drops. The polymer of the contact lens acts as a barrier or reservoir and facilitates the sustained release of drugs for a prolonged period, which diminishes the need for multiple doses and provides enhanced bioavailability. As a topical delivery system, the drug-laden CLs reduce the systemic absorption and circulation of the drug, thereby reducing the systemic side effects [108]. The simple soaking technique provides poor loading attributed to the very small drug concentration gradient. Poly-(cyclo)dextrins are suggested to enhance drug loading in CLs by increasing the concentration of the drug in the aqueous phase, which in turn may also increase distinct drug network interactions [111, 112].
Contact lenses offer the significant advantage of improved ocular bioavailability, from < 5% of eye drops to ~ 50%, which is attributed to improved precorneal retention. However, the drug uptake mechanism and release kinetics of conventional lenses and silicone-based lenses are not well understood. Phan et al. studied the in vitro uptake and release kinetics of Nat from the different commercially available conventional hydrogel lenses (CHL) and silicone hydrogel lenses (SiHL). Although both CHL and SiHL released clinically significant drug, they suffered from burst release, suggesting their non-suitability for commercial use for the management of fungal keratitis [41]. Extensive research needs to be directed towards the investigation of CL material to impart sustained drug release. Several researchers are exploring different techniques to address the poor drug loading and burst release from contact lens. The major method employed for drug loading is CLs is a simple soaking technique, which suffers from poor drug loading and burst release. To overcome the challenges, Phan et al. explored cyclodextrin functionalized contact lens material for both conventional hydrogel and silicone-based lenses. The study suggested CDs' concentration-based improved drug loading, and drug release from CDs functionalized methacrylated β-CD and methacrylated HP-β-CD CL material. Higher CD concentration may provide an unfavorable arrangement of CDs, limiting the binding of the drug with CDS [113].
Ocular Inserts
These can be defined as drug-loaded, thin, sterile, multilayered, solid or semi-solid consistency devices that are specifically designed (size and shape) to be placed in the lower or upper fornix or cornea [114, 115]. These ocular devices can be formulated using insoluble polymers or soluble natural materials and can be developed as mucoadhesive or even soluble ocular drug inserts [116]. The major benefit of the inserts is having a high precorneal residence time, which provides a means to release the drug material at a preprogrammed rate [117] ultimately leading to higher bioavailability and prolonged drug activity. There is a decrease in systemic absorption, reduction in dosing frequency and opportunity to target the internal eye tissues. Issues like difficulty in application or eye irritation due to rigidity lead to a reduction in patient compliance [115, 118].
Bhandari et al. incorporated Nat solid dispersion into a polymeric ophthalmic film [polyvinyl alcohol (PVA), chitosan and ethyl cellulose] to increase the retention time at the site, provide sustained release and improve Nat bioavailability. In vitro permeation studies showed that with an increase in the concentration of the polymers, Nat release decreased, which may be attributed to the longer drug diffusion pathway. The ex vivo permeation study showed a lower drug permeation than the in vitro, perhaps due to the varying pore size of the cornea and some of the fatty tissues that prevent the drug permeation. An inhibition zone that was sustained for 24 h and maintained for up to 15 days was observed in the antimicrobial test (cup-let method), and the Draize eye irritation test revealed that the formulation was non-irritant [119].
Rajasekaran et al. designed controlled-release Nat polymeric ocular inserts using various proportions of Eudragit (L-100, S-100 and RL-100), hydroxypropyl methylcellulose phthalate and cellulose acetate phthalate with PEG-400 as a plasticizer. A formulation with 3% Eudragit RL-100 and 1% Eudragit L-100 showed minimum moisture absorption due to the hydrophobic molecular substitutions of Eudragit RL-100, which showed zero-order drug release with an extended Nat release period of 23 h [120].
Patents
Currently, there are two patents for the commercial production of Nat synthesized by fermentation using either of the two species, Streptomyces natalensis or Streptomyces gilvosporeus, and the processes described by both are similar [13, 26]. One patent was filed by inventors Bertus Noordam, Jacobus Stark, Ben R. De Haan and Hong Sheng Tan (patent number: US5552151) for stable natamycin suspensions, filed on April 11, 1995, and granted on September 3, 1996, which was assigned to Gist-brocades B.V. and granted by the US Patent and Trademark Office (USPTO). These are concentrated suspensions of Nat, which show good physical, chemical and microbial stability (> 14 days) and are suitable as stock suspensions for synthesizing immersion liquids and coating emulsions for treating food, feed and agricultural products. The suspension contains thickening agents (methylcellulose, carrageenan gum, Arabic gum, xanthan gum and their combinations) and is maintained at a pH range of 3–4.8 without the addition of any preservation [143].
Currently, a patent on Natasol, a sterile water-soluble intrastromal natamycin (IS-NTM) of 10 μg/0.1 ml, soluble Nat prepared by the Ocular Pharmacology and Pharmacy Division of AIIMS, New Delhi, India, as an adjunct for topical therapy for recalcitrant filamentous fungal keratitis is pending [144, 145] summarized in Table 4.
Conclusion and Future Prospects
The incidence of ocular fungal keratitis has been increasing, and conventional topical dosage forms offer poor management owing to the physiologic and anatomical barriers present in ocular delivery. Recent developments in Nat ocular delivery have increased the potential for efficient drug delivery over time, with increased drug retention times resulting in improved bioavailability and antifungal efficacy. Much research has been done on Nat delivery; however, no alternative dosage forms have reached the clinical setting. This may be attributed to economics (intensive investment in resources), physicochemical parameters, failure to scale up pilot studies (advanced process designs) and limited understanding of long-term toxicity. Most of the topical nanoformulations show an initial burst release, which may lead to increased nonspecific absorption. Another major challenge in marketing these formulations is the sterilization and counter-effect they have on the various physicochemical parameters of nanocarriers such as size, polydispersity index and stability; currently, none of the researchers have studied these effects on their formulation.
Furthermore, Nat is reported to undergo decomposition when exposed to ultraviolet or gamma radiation owing to the loss of the tetraene structure. However, it is not inactivated by visible light unless there is a transfer of photoenergy. The photodegradation products contain a tetraene chromophore, which is different from the parent Nat. Most of the current research focuses on addressing the solubility and dose frequency issues of Nat, but more research into the drug’s photostability is needed. For clinical approval of these formulations, an extensive study must be done on the stability, long-term toxicity and controlled drug release.
References
Hoffman JJ, Burton MJ, Leck A, Hoffman C, Burton JJ, Leck MJ et al. Mycotic keratitis—a global threat from the filamentous fungi. J Fungi 2021;7(4): 273. https://www.mdpi.com/2309-608X/7/4/273/htm.
Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021;21(3):e49-57.
Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002;21(6):555–9. https://pubmed.ncbi.nlm.nih.gov/12131029/.
Mahmoudi S, Masoomi A, Ahmadikia K, Tabatabaei SA, Soleimani M, Rezaie S, et al. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. 2018;61(12):916–30. https://pubmed.ncbi.nlm.nih.gov/29992633/.
Qiao GL, Ling J, Wong T, Yeung SN, Iovieno A. Candida keratitis: epidemiology, management, and clinical outcomes. Cornea. 2020;39(7):801–5. https://pubmed.ncbi.nlm.nih.gov/32265382/.
Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–20. https://pubmed.ncbi.nlm.nih.gov/23398543/.
Jain A, Shah SG, Chugh A. Cell penetrating peptides as efficient nanocarriers for delivery of antifungal compound, natamycin for the treatment of fungal keratitis. Pharm Res. 2015;32(6):1920–30. https://doi.org/10.1007/s11095-014-1586-x.
Qiu S, Zhao GQ, Lin J, Wang X, Hu LT, Du ZD, et al. Natamycin in the treatment of fungal keratitis: a systematic review and Meta-analysis. Int J Ophthalmol. 2015;8(3):597–602. https://pubmed.ncbi.nlm.nih.gov/26086015/.
Bouaoud C, Xu S, Mendes E, Lebouille JGJL, De Braal HEA, Meesters GMH. Development of biodegradable polymeric nanoparticles for encapsulation, delivery, and improved antifungal performance of natamycin. J Appl Polym Sci. 2016. https://doi.org/10.1002/app.43736.
Liu Y, Cui X, Zhao L, Zhang W, Zhu S, Ma J. Chitosan nanoparticles to enhance the inhibitory effect of natamycin on candida albicans. J Nanomater. 2021. https://doi.org/10.1155/2021/6644567.
Venkatesh Prajna N, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422. /pmc/articles/PMC3769211/.
Spierer O, Dugar J, Miller D, O’Brien TP. Comparative antifungal susceptibility analysis of Candida albicans versus non-albicans Candida corneal isolates. Cornea. 2015;34(5):576–9. https://journals.lww.com/corneajrnl/Fulltext/2015/05000/Comparative_Antifungal_Susceptibility_Analysis_of.17.aspx.
Brik H. Analytical profiles of drug substances. In: Florey K, editor. Natamycin. New York: Academic Press, Inc; 1981. p. 513–61.
Stark J. PRESERVATIVES | Permitted preservatives—natamycin. In: Robinson R, Bhatt C, Patel P, editors. Encyclopedia of food microbiology. San Diego: Academic Press; 1999. p. 1776–81.
Brik H. Natamycin. Anal Profiles Drug Subst Excipients. 1981;10(1):513–61.
BRIK H. New high-molecular decomposition products of natamycin (pimaricin) with intact lactone-ring. J Antibiot (Tokyo). 1976;29(6):632–7. http://joi.jlc.jst.go.jp/JST.Journalarchive/antibiotics1968/29.632?from=CrossRef.
Dekker J, Ark PA. Protection of antibiotic pimaricin from oxidation and ultraviolet light by chlorophyllin and other compounds. Antibiot Chemother (Northfield, Ill). 1959;9(6):327–32. http://www.ncbi.nlm.nih.gov/pubmed/24545263.
Thomas PA. Fungal infections of the cornea. Eye. 2003;17(8):852–62. http://www.nature.com/articles/6700557.
Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep. 2013;7(3):209–18. https://pubmed.ncbi.nlm.nih.gov/24040467/.
Kaur IP, Rana C, Singh H. Development of effective ocular preparations of antifungal agents. J Ocul Pharmacol Ther. 2008;24(5):481–93. https://pubmed.ncbi.nlm.nih.gov/18788998/.
Lalitha P, Shapiro BL, Srinivasan M, Prajna NV, Acharya NR, Fothergill AW, et al. Antimicrobial susceptibility of fusarium, aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125(6):789–93. https://jamanetwork.com/journals/jamaophthalmology/fullarticle/419410.
Sharma S, Das S, Virdi A, Fernandes M, Sahu SK, Koday NK, et al. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol. 2015;99(9):1190–5. https://pubmed.ncbi.nlm.nih.gov/25740805/.
Cordeiro RA, Teixeira CEC, Brilhante RSN, Castelo-Branco DSCM, Paiva MAN, Giffoni Leite JJ, et al. Minimum inhibitory concentrations of amphotericin B, azoles and caspofungin against Candida species are reduced by farnesol. Med Mycol. 2013;51(1):53–9. https://academic.oup.com/mmy/article/51/1/53/976235.
Pfaller MA, Andes D, Arendrup MC, Diekema DJ, Espinel-Ingroff A, Alexander BD, et al. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis. 2011;70(3):330–43.
Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci USA. 2012;109(7):2234–9. https://doi.org/10.1073/pnas.1117280109.
Patil A, Lakhani P, Majumdar S. Current perspectives on natamycin in ocular fungal infections. J Drug Deliv Sci Technol. 2017;1(41):206–12.
In vitro evaluation of natamycin 5% suspension against Aspergillus flavus, Fusarium solani, and Candida parasilopsis – Philippine. J Ophthalmol. https://paojournal.com/article/in-vitro-evaluation-of-natamycin-5-suspension-against-aspergillus-flavus-fusarium-solani-and-candida-parasilopsis/.
Córdoba S, Rodero L, Vivot W, Abrantes R, Davel G, Vitale RG. In vitro interactions of antifungal agents against clinical isolates of Fusarium spp. Int J Antimicrob Agents. 2008;31(2):171–4.
Al-Hatmi AMS, Meletiadis J, Curfs-Breuker I, Bonifaz A, Meis JF, De Hoog GS. In vitro combinations of natamycin with voriconazole, itraconazole and micafungin against clinical Fusarium strains causing keratitis. J Antimicrob Chemother. 2016;71(4):953–5. https://academic.oup.com/jac/article/71/4/953/2363794.
Arikan S, Lozano-Chiu M, Paetznick V, Nangia S, Rex JH. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of aspergillus and fusarium species. J Clin Microbiol. 1999;37(12):3946–51. https://doi.org/10.1128/JCM.37.12.3946-3951.1999.
Brothers AM, Wyatt RD. The antifungal activity of natamycin toward molds isolated from commercially manufactured poultry feed. Avian Dis. 2000;44(3):490–7.
Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16(4):730. /pmc/articles/PMC207127/.
O’Day DM. Selection of appropriate antifungal therapy. Cornea. 1987;6(4):238–45. https://pubmed.ncbi.nlm.nih.gov/3319407/.
Loh AR, Hong K, Lee S, Mannis M, Acharya NR. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28(8):856–9. https://pubmed.ncbi.nlm.nih.gov/19654533/.
Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–8. https://pubmed.ncbi.nlm.nih.gov/16935335/.
Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63(3):333–8. https://pubmed.ncbi.nlm.nih.gov/4820359/.
Shell JW. Pharmacokinetics of topically applied ophthalmic drugs. Surv Ophthalmol. 1982;26(4):207–18. https://pubmed.ncbi.nlm.nih.gov/7041308/.
USFDA. NDA 50–514/S-009 Page 3 NATACYN1 (natamycin ophthalmic suspension). 2008. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/%0A050514s009lbl.pdf.
Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74(8):477. /pmc/articles/PMC1042177/?report=abstract.
Rotchford AP, Murphy KM. Compliance with timolol treatment in glaucoma. Eye (Lond). 1998;12(Pt 2)(2):234–6. https://pubmed.ncbi.nlm.nih.gov/9683946/.
Phan CM, Subbaraman LN, Jones L. In vitro uptake and release of natamycin from conventional and silicone hydrogel contact lens materials. Eye Contact Lens. 2013;39(2):162–8. https://pubmed.ncbi.nlm.nih.gov/23392304/
Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm. 2013;39(11):1599–617. https://pubmed.ncbi.nlm.nih.gov/23153114/.
Gause S, Hsu KH, Shafor C, Dixon P, Powell KC, Chauhan A. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv Colloid Interface Sci. 2016;233:139–54. https://pubmed.ncbi.nlm.nih.gov/26318359/.
Johns KJ, O’Day DM. Pharmacologic management of keratomycoses. Surv Ophthalmol. 1988;33(3):178–88. https://pubmed.ncbi.nlm.nih.gov/3068821/.
Bhatta RS, Chandasana H, Rathi C, Kumar D, Chhonker YS, Jain GK. Bioanalytical method development and validation of natamycin in rabbit tears and its application to ocular pharmacokinetic studies. J Pharm Biomed Anal. 2011;54(5):1096–100.
Hosny KM, Rizg WY, Alkhalidi HM, Abualsunun WA, Bakhaidar RB, Almehmady AM, et al. Nanocubosomal based in situ gel loaded with natamycin for ocular fungal diseases: development, optimization, in-vitro, and in-vivo assessment. Drug Deliver. 2021;28(1):1836–48. https://doi.org/10.1080/10717544.2021.1965675.
Nagarwal RC, Kumar R, Pandit JK. Chitosan coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: in vitro characterization and in vivo study in rabbit eye. Eur J Pharm Sci. 2012;47(4):678–85. https://pubmed.ncbi.nlm.nih.gov/22922098/
Omerović N, Vranić E. Application of nanoparticles in ocular drug delivery systems. Heal Technol. 2019;10(1):61–78. https://doi.org/10.1007/s12553-019-00381-w.
Battaglia L, Serpe L, Foglietta F, Muntoni E, Gallarate M, Del Pozo Rodriguez A, et al. Application of lipid nanoparticles to ocular drug delivery. Expert Opin Drug Deliv. 2016;13(12):1743–57. https://pubmed.ncbi.nlm.nih.gov/27291069/
Chandasana H, Prasad YD, Chhonker YS, Chaitanya TK, Mishra NN, Mitra K, et al. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: an approach to reduce dose and dosing frequency. Int J Pharm. 2014;477(1–2):317–25. https://pubmed.ncbi.nlm.nih.gov/25455776/
Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int J Pharm. 2012;432(1–2):105–12.
Sha X, Chan L, Fan X, Guo P, Chen T, Liu L, et al. Thermosensitive tri-block polymer nanoparticle-hydrogel composites as payloads of natamycin for antifungal therapy against fusarium solani. Int J Nanomedicine. 2022;17:1463. /pmc/articles/PMC8976233/
Balguri SP, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur J Pharm Biopharm. 2016;1(109):224–35.
Singh A, Ubrane R, Prasad P, Ramteke S. Preparation and characterization of rizatriptan benzoate loaded solid lipid nanoparticles for brain targeting. Mater Today Proc. 2015;2(9):4521–43.
Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int. 2014;2014. https://pubmed.ncbi.nlm.nih.gov/24995293/.
Askarizadeh A, Barreto GE, Henney NC, Majeed M, Sahebkar A. Neuroprotection by curcumin: a review on brain delivery strategies. Int J Pharm. 2020;30(585): 119476.
Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy AOH. Natamycin solid lipid nanoparticles – sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomed. 2019;14:2515. /pmc/articles/PMC6459158/.
Abdelmonem R, El-Nabarawi MA, Attia AM, Teaimaa M. Ocular delivery of natamycin solid lipid nanoparticle loaded mucoadhesive gel: formulation, characterization and in vivo study. Int J Appl Pharm. 2020;12(5):173–80. https://innovareacademics.in/journals/index.php/ijap/article/view/38824/23591.
Agrawal M, Saraf S, Pradhan M, Patel RJ, Singhvi G, Ajazuddin, et al. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed Pharmacother. 2021;141: 111919.
Akel H, Ismail R, Csóka I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur J Pharm Biopharm. 2020;1(148):38–53.
Balguri SP, Adelli G, Bhagav P, Repka MA, Majumdar S. Development of nano structured lipid carriers of ciprofloxacin for ocular delivery: characterization, in vivo distribution and effect of PEGylation. Invest Ophthalmol Vis Sci. 2015;56(7):2269–2269.
Jiang W, Wang J, Yang L, Jiang X, Bai Z, Wang Z, et al. Nanostructured lipid carriers modified with PEGylated carboxymethylcellulose polymers for effective delivery of docetaxel. RSC Adv. 2015;5(110):90386–95. https://pubs.rsc.org/en/content/articlehtml/2015/ra/c5ra13642c.
Patil A, Lakhani P, Taskar P, Wu KW, Sweeney C, Avula B, et al. Formulation development, optimization, and in vitro-in vivo characterization of natamycin-loaded pegylated nano-lipid carriers for ocular applications. J Pharm Sci. 2018;107(8):2160–71. https://pubmed.ncbi.nlm.nih.gov/29698725/.
Rageeb Md Usman M, Vijaykumar Jain B, Ghuge PR, Jain B V. Niosomes: a novel trend of drug delivery. Eur J Biomed Pharm Sci. 2017;4(7):436–42. https://www.researchgate.net/profile/Md-Usman/publication/318113949_Niosomes_A_Novel_Trend_of_Drug_Delivery/links/598b1990a6fdcc7cf926ebc1/Niosomes-A-Novel-Trend-of-Drug-Delivery.pdf.
El-Nabarawi MA, Abd El Rehem RT, Teaima M, Abary M, El-Mofty HM, Khafagy MM, et al. Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies. Drug Dev Ind Pharm. 2019;45(6):922–36. https://doi.org/10.1080/03639045.2019.1579827.
Paecharoenchai O, Teng L, Yung BC, Teng L, Opanasopit P, Lee RJ. Nonionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine (Lond). 2013;8(11):1865. /pmc/articles/PMC3971008/
Arumugam K, Payal B, Jitendra V S, Rajashree C, Govind S. Niosomes: a novel carrier drug delivery system. J Drug Deliv Ther. 2021;11(1):162–70. http://jddtonline.info/index.php/jddt/article/view/4479.
Verma A, Jain A, Tiwari A, Jain SK. Preformulation considerations of Natamycin and development of Natamycin loaded niosomal formulation. Asian J Pharm Pharmacol. 2019;5(5):1022–30.
Verma A, Jain A, Tiwari A, Saraf S, Panda PK, Jain SK. Promising antifungal potential of engineered non-ionic surfactant-based vesicles: in vitro and in vivo studies. AAPS PharmSciTech. 2021;22(1):1–14. https://doi.org/10.1208/s12249-020-01900-z.
Paradkar MU, Parmar M. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. J Drug Deliv Sci Technol. 2017;1(39):113–22.
Gupta P, Mazumder R, Padhi S. Glycerosomes: advanced liposomal drug delivery system. Indian J Pharm Sci. 2020;82(3):385–97. https://www.ijpsonline.com/articles/glycerosomes-advanced-liposomal-drug-delivery-system-3921.html.
Salem HF, Kharshoum RM, Sayed OM, Abdel Hakim LF. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box-Behnken statistical design. Drug Dev Ind Pharm. 2018;44(11):1871–84. https://doi.org/10.1080/03639045.2018.1504963.
Naguib MJ, Hassan YR, Abd-Elsalam WH. 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int J Pharm. 2021;25(607): 121010.
Gupta P, Mazumder R, Padhi S, Gupta MP, Pharm M. Development of Natamycin loaded glycerosomes-a novel approach to defend ophthalmic keratitis. Indian J Pharm Educ Res 2020;54. www.ijper.org
Ahmed S, Kassem MA, Sayed S. Bilosomes as promising nanovesicular carriers for improved transdermal delivery: construction, in vitro optimization, ex vivo permeation and in vivo evaluation. Int J Nanomedicine. 2020;15:9783. /pmc/articles/PMC7733410/
Abdelbary AA, Abd-Elsalam WH, Al-mahallawi AM. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int J Pharm. 2016;513(1–2):688–96.
Mohsen AM, Salama A, Kassem AA. Development of acetazolamide loaded bilosomes for improved ocular delivery: preparation, characterization and in vivo evaluation. J Drug Deliv Sci Technol. 2020;1(59): 101910.
Janga KY, Tatke A, Balguri SP, Lamichanne SP, Ibrahim MM, Maria DN, et al. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: in vitro permeability, cytotoxicity and in vivo evaluation. Artif Cells Nanomed Biotechnol. 2018;46(sup1):1039–50. https://doi.org/10.1080/21691401.2018.1443117.
Walve JR, Bakliwal SR, Rane BRPSP. Transfersomes: a surrogated carrier for transdermal drug delivery system. Int J Appl Biol Pharm Technol. 2011;2(1):204–13.
Modi C, Bharadia P. Transferosomes: new dominants for ransdermal drug delivery. Am J Pharmtech Res. 2012;2(3):71–91.
Pahwa R, Pal S, Saroha K, Waliyan P, Kumar M. Transferosomes: unique vesicular carriers for effective transdermal delivery. J Appl Pharm Sci. 2021;11(5):1–8.
Hussain A, Singh S, Sharma D, Webster TJ, Shafaat K, Faruk A. Elastic liposomes as novel carriers: recent advances in drug delivery. Int J Nanomedicine. 2017;12:5087. /pmc/articles/PMC5522681/.
Rai S, Pandey V, Rai G, Pharmaceutics R, Khasla R. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: the state of the art. Nano Rev Exp. 2017;8(1):1325708. https://doi.org/10.1080/20022727.2017.1325708.
Janga KY, Tatke A, Dudhipala N, Balguri SP, Ibrahim MM, Maria DN, et al. Gellan gum based sol-to-gel transforming system of natamycin transfersomes improves topical ocular delivery. J Pharmacol Exp Ther. 2019;370(3):814–22. https://jpet.aspetjournals.org/content/370/3/814
Gaballa SA, El Garhy OH, Abdelkader H. Cubosomes: composition, preparation, and drug delivery applications. J Adv Biomed Pharm Sci. 2020;3(1):1–9. https://jabps.journals.ekb.eg/article_54781.html
Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov Today. 2016;21(5):789–801.
Younes NF, Abdel-Halim SA, Elassasy AI. Corneal targeted Sertaconazole nitrate loaded cubosomes: Preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int J Pharm. 2018;553(1–2):386–97. https://pubmed.ncbi.nlm.nih.gov/30393167/.
Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm. 2013;39(11):1599–617. https://doi.org/10.3109/03639045.2012.736515.
Kazi M, Dhakne R, Dehghan MH. Ocular delivery of natamycin based on monoolein/span 80/poloxamer 407 nanocarriers for the effectual treatment of fungal keratitis. 2020; https://doi.org/10.35333/jrp.2020.142.
Figueroa-Ochoa EB, Villar-Alvarez EM, Cambón A, Mistry D, Llovo J, Attwood D, et al. Lenghty reverse poly(butylene oxide)-poly(ethylene oxide)-poly(butylene oxide) polymeric micelles and gels for sustained release of antifungal drugs. Int J Pharm. 2016;510(1):17–29. https://www.researchgate.net/publication/303917608_Lenghty_reverse_polybutylene_oxide-polyethylene_oxide-polybutylene_oxide_polymeric_micelles_and_gels_for_sustained_release_of_antifungal_drugs.
Durgun ME, Güngör S, Özsoy Y. Micelles: promising ocular drug carriers for anterior and posterior segment diseases. J Ocul Pharmacol Ther. 2020;36(6):323–41. https://pubmed.ncbi.nlm.nih.gov/32310723/.
Li Z, Liu M, Ke L, Wang LJ, Wu C, Li C, et al. Flexible polymeric nanosized micelles for ophthalmic drug delivery: research progress in the last three years. Nanoscale Adv. 2021;3(18):5240–54. https://pubs.rsc.org/en/content/articlehtml/2021/na/d1na00596k.
Zhou T, Zhu L, Xia H, He J, Liu S, He S, et al. Micelle carriers based on macrogol 15 hydroxystearate for ocular delivery of terbinafine hydrochloride: In vitro characterization and in vivo permeation. Eur J Pharm Sci. 2017;15(109):288–96.
Guo Y, Karimi F, Fu Q, Qiao G, Zhang H. Reduced administration frequency for the treatment of fungal keratitis: a sustained natamycin release from a micellar solution. Expert Opin Drug Delive. 2020;17(3):407–21. https://doi.org/10.1080/17425247.2020.1719995.
Lorenzo-Veiga B, Sigurdsson HH, Loftsson T, Alvarez-Lorenzo C. Cyclodextrin–amphiphilic copolymer supramolecular assemblies for the ocular delivery of natamycin. Nanomater 2019;9(5):745. https://www.mdpi.com/2079-4991/9/5/745/htm.
Pescina S, Ostacolo C, Gomez-Monterrey IM, Sala M, Bertamino A, Sonvico F, et al. Cell penetrating peptides in ocular drug delivery: state of the art. J Control Release. 2018;284:84–102. https://europepmc.org/article/med/29913221.
Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–93. https://pubmed.ncbi.nlm.nih.gov/2849510/.
Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008;16(1):107–14. https://pubmed.ncbi.nlm.nih.gov/17923842/.
Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. 2017;38(4):406–24.
Rohira H, Shankar S, Yadav S, Shah SG, Chugh A. Enhanced in vivo antifungal activity of novel cell penetrating peptide natamycin conjugate for efficient fungal keratitis management. Int J Pharm. 2021;1(600): 120484.
Romero GB, Keck CM, Müller RH, Bou-Chacra NA. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;1(107):215–22.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160(3):418–30. https://pubmed.ncbi.nlm.nih.gov/22465393/.
Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183(1):51–66.
Koland M, Das R, Sindhoor SM. Design and Evaluation of Natamycin Nanocrystals Loaded In Situ Gel for Ophthalmic Administration. Artic J Pharm Res Int. 2021;33(38A):307–24. https://www.sdiarticle4.com/review-history/71391.
Da Silva GR, Lima TH, Fernandes-Cunha GM, Oréfice RL, Da Silva-Cunha A, Zhao M, et al. Ocular biocompatibility of dexamethasone acetate loaded poly(ɛ-caprolactone) nanofibers. Eur J Pharm Biopharm. 2019;1(142):20–30.
Deepak A, Goyal AK, Rath G. Nanofiber in transmucosal drug delivery. J Drug Deliv Sci Technol. 2018;1(43):379–87.
Fonseca Veras F, Ana ·, Ritter C, Roggia I, Pranke P, Cláudio, et al. Natamycin-loaded electrospun poly(ε-caprolactone) nanofibers as an innovative platform for antifungal applications. SN Appl Sci. 123AD;2. https://doi.org/10.1007/s42452-020-2912-z
Siafaka PI, Pınar Yağcilar A, Üstündağ Okur N. New era of ocular drug delivery systems based on contact lenses. J Pharm Sci. 2020;45:161–74.
McNamara NA, Polse KA, Brand RJ, Graham AD, Chan JS, McKenney CD. Tear mixing under a soft contact lens: effects of lens diameter. Am J Ophthalmol. 1999;127(6):659–65. https://pubmed.ncbi.nlm.nih.gov/10372875/.
Grassiri B, Zambito Y, Bernkop-Schnürch A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv Colloid Interface Sci. 2021;1(288): 102342.
García-Fernández MJ, Tabary N, Martel B, Cazaux F, Oliva A, Taboada P, et al. Poly-(cyclo)dextrins as ethoxzolamide carriers in ophthalmic solutions and in contact lenses. Carbohydr Polym. 2013;98(2):1343–52.
Koontz J, chemistry JM-J of agricultural and food, 2003 undefined. Formation of natamycin: cyclodextrin inclusion complexes and their characterization. ACS Publ. https://doi.org/10.1021/jf030332y.
Phan CM, Subbaraman LN, Jones L. In vitro drug release of natamycin from β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin-functionalized contact lens materials. J Biomater Sci Polym Edition. 2014;25(17):1907–19. https://doi.org/10.1080/09205063.2014.958016.
Grass GM, Cobby J, Makoid MC. Ocular delivery of pilocarpine from erodible matrices. J Pharm Sci. 1984;73(5):618–21. https://pubmed.ncbi.nlm.nih.gov/6737234/.
Polat HK, Kurt N, Aytekin E, Bozdaǧ Pehlivan S, Çallş S. Novel drug delivery systems to improve the treatment of keratitis. J Ocul Pharmacol Ther. 2022;38(6):376–95. https://doi.org/10.1089/jop.2021.0127.
Morrison PWJ, Khutoryanskiy VV. Advances in ophthalmic drug delivery. Ther Deliv. 2014;5(12):1297–315. https://doi.org/10.4155/tde.14.75.
Patel M, Patel M, Patel D. Formulation and evaluation of drug-free ophthalmic films prepared by using various synthetic polymers. J Young Pharm. 2009;1(2):116. http://www.jyoungpharm.in/text.asp?2009/1/2/116/55742.
Saettone MF, Salminen L. Ocular inserts for topical delivery. Adv Drug Deliv Rev. 1995;16(1):95–106.
Bhandari L, Patil AS, Bolmal U, Masareddy R, Dandagi P. Formulation and evaluation of natamycin solid dispersion incorporated ophthalmic films. Indian J Pharm Educ Res;56. www.ijper.org.
Rajasekaran A, Sivakumar V, Karthika K, Preetha JP, Abirami T. Design and evaluation of polymeric controlled release natamycin ocular inserts. Kathmandu Univ J Sci Eng Technol. 2010;6(1):108–15. https://www.nepjol.info/index.php/KUSET/article/view/3318.
Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3(2):234–58. http://www.ncbi.nlm.nih.gov/pubmed/10994037.
Müller RH, Maaßen S, Weyhers H, Mehnert W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J Drug Target. 1996;4(3):161–70. https://pubmed.ncbi.nlm.nih.gov/8959488/.
Wake MC, Gerecht PD, Lu L, Mikos AG. Effects of biodegradable polymer particles on rat marrow-derived stromal osteoblasts in vitro. Biomaterials. 1998;19(14):1255–68. https://pubmed.ncbi.nlm.nih.gov/9720889/.
Battaglia L, Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv. 2012;9(5):497–508. https://pubmed.ncbi.nlm.nih.gov/22439808/.
Battaglia L, Serpe L, Foglietta F, Muntoni E, Gallarate M, Del Pozo Rodriguez A, et al. Application of lipid nanoparticles to ocular drug delivery. Expert Opin Drug Deliv. 2016;13(12):1743–57. https://pubmed.ncbi.nlm.nih.gov/27291069/.
Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1–2):121–8. https://pubmed.ncbi.nlm.nih.gov/12176234/.
Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1–2):1–17.
Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2008;70(2):633–40. https://pubmed.ncbi.nlm.nih.gov/18577447/.
Gan L, Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov Today. 2013;18(5–6):290–7. https://pubmed.ncbi.nlm.nih.gov/23092895/.
Zhang K, Zhang Y, Li Z, Li N, Feng N. Essential oil-mediated glycerosomes increase transdermal paeoniflorin delivery: optimization, characterization, and evaluation in vitro and in vivo. Int J Nanomedicine. 2017;12:3521–32. https://pubmed.ncbi.nlm.nih.gov/28503066/.
Aburahma MH. Bile salts-containing vesicles: promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. 2014;23(6):1847–67. https://doi.org/10.3109/10717544.2014.976892.
Tilekar K, Khade P, Sujit Kakade SK. Cubosomes- A drug delivery system. Int J Pharm Chem Biol Sci. 2014;4(4):812–24.
Douwe D, Breimer PS, editors. Topics in Pharmaceutical Sciences. 57th ed. New York: Elsevier Science Publishers; 1985. p. 291.
Lu Y, Zhang E, Yang J, Cao Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018;11(10):4985. /pmc/articles/PMC6201237/.
Buccini DF, Cardoso MH, Franco OL. Antimicrobial peptides and cell-penetrating peptides for treating intracellular bacterial infections. Front Cell Infect Microbiol. 2020;10. /pmc/articles/PMC7892433/.
Budagavi DP, Chugh A. Antibacterial properties of Latarcin 1 derived cell-penetrating peptides. Eur J Pharm Sci. 2018;115:43–9. https://pubmed.ncbi.nlm.nih.gov/29329747/.
Kristensen M, Birch D, Nielsen HM. Applications and challenges for use of cell-penetrating peptides as delivery vectors for peptide and protein cargos. Int J Mol Sci. 2016;17(2). /pmc/articles/PMC4783919/.
Müller RH, Gohla S, Keck CM. State of the art of nanocrystals—special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78(1):1–9.
Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev. 2011;17(5):349. /pmc/articles/PMC3179616/.
Muntz A, Subbaraman LN, Sorbara L, Jones L. Tear exchange and contact lenses: a review. J Optom. 2015;8(1):2–11. https://linkinghub.elsevier.com/retrieve/pii/S1888429614000995.
Kakisu K, Matsunaga T, Kobayakawa S, Sato T, Tochikubo T. Development and efficacy of a drug-releasing soft contact lens. Investig Opthalmology Vis Sci. 2013;54(4):2551. https://doi.org/10.1167/iovs.12-10614.
L. Lanier O, Christopher KG, Macoon RM, Yu Y, Sekar P, Chauhan A. Commercialization challenges for drug eluting contact lenses. Expert Opin Drug Deliv. 2020;17(8):1133–49. https://pubmed.ncbi.nlm.nih.gov/32602822/.
US5552151A—Stable natamycin suspensions—Google Patents. https://patents.google.com/patent/US5552151A/en?oq=US5552151.
Saluja G, Sharma N, Agarwal R, Sharma HP, Maharana P, Satpathy G, et al. Determination of surgical outcomes with a novel formulation of intrastromal natamycin in recalcitrant fungal keratitis: a pilot study. Indian J Ophthalmol. 2021;69(10):2670. /pmc/articles/PMC8597472/
Velpandian T, Nirmal J, Sharma HP, Sharma S, Sharma N, Halder N. Novel water soluble sterile natamycin formulation (Natasol) for fungal keratitis. Eur J Pharm Sci. 2021;1(163): 105857.
Acknowledgements
Funding
Open Access funding provided by Manipal Academy of Higher Education, Manipal. No funding or sponsorship was received for publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
Author Contributions
Mabel Mascarenhas: Writing—Original draft, review and editing. Pinal Chaudhari: Conceptualization, Visualization, review and editing. Shaila A. Lewis: Conceptualization, Supervision, review and editing. All the authors made substantial contribution to the conception of the article, reviewed the article and approved the final version of the manuscript.
Disclosures
Mabel Mascarenhas, Pinal Chaudhari and Shaila A. Lewis confirm that they have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mascarenhas, M., Chaudhari, P. & Lewis, S.A. Natamycin Ocular Delivery: Challenges and Advancements in Ocular Therapeutics. Adv Ther 40, 3332–3359 (2023). https://doi.org/10.1007/s12325-023-02541-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02541-x