Abstract
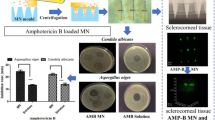
Fungal keratitis (FK), the major cause of ocular morbidity worldwide, is commonly treated with the amphotericin B (AmB) eye drops extemporaneously prepared from marketed parenteral formulations. However, these in-house prepared AmB eye drops have the drawbacks of poor ocular bioavailability and eye irritation. The aim of this study was to develop AmB-loaded fibroin nanoparticles (AmB-FNPs), in combination with the polymer PEG 400, as ready-to-use eye drops for FK. The AmB-FNPs were prepared by desolvation method. All AmB-FNPs exhibited homogeneous spherical particles with a mean size of ~ 270 nm, the zeta potential of ~ − 17 mV, and an entrapment efficiency of ~ 65%. Using X-ray diffraction and UV analysis, AmB demonstrated amorphous molecular dispersion and monomeric form when entrapped in the FNPs. Interestingly, in dissolution studies, although AmB-FNPs showed no detectable drug release in sink condition, they still possessed good antifungal activity against Candida albicans. Potentially, AmB-FNPs showed less cytotoxicity in human corneal epithelial cell line compared to the marketed AmB deoxycholate.






Similar content being viewed by others
References
Gallis HA, Drew RH, Pickard WW (1990) Amphotericin B: 30 years of clinical experience. Rev Infect Dis 12(2):308–329
Tuli SS (2011) Fungal keratitis. Clin Ophthalmol 5:275–279
Thomas PA, Kaliamurthy J (2013) Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 19(3):210–220
Chhonker YS, Prasad YD, Chandasana H, Vishvkarma A, Mitra K, Shukla PK, Bhatta RS (2015) Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int J Biol Macromol 72:1451–1458
Morand K, Bartoletti AC, Bochot A, Barratt G, Brandely ML, Chast F (2007) Liposomal amphotericin B eye drops to treat fungal keratitis: physico-chemical and formulation stability. Int J Pharm 344(1–2):150–153
Fu T, Yi J, Lv S, Zhang B (2017) Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J Liposome Res 27(3):228–233
Suresh PK, Sah AK (2014) Nanocarriers for ocular delivery for possible benefits in the treatment of anterior uveitis: focus on current paradigms and future directions. Expert Opin Drug Deliv 11(11):1747–1768
Baranowski Przemysław, Karolewicz Bożena, Gajda Maciej, Pluta J (2014) Ophthalmic drug dosage forms: characterisation and research methods. Sci World J 2014:14
Das S, Suresh PK (2011) Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomedicine 7(2):242–247
Gratieri T, Gelfuso GM, Lopez RFV, Souto EB (2010) Current efforts and the potential of nanomedicine in treating fungal keratitis. Expert Rev Ophthalmol 5(3):365–384
Sharma A, Taniguchi J (2017) Review: emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul Surf 15(4):670–679
Zhou W, Wang Y, Jian J, Song S (2013) Self-aggregated nanoparticles based on amphiphilic poly(lactic acid)-grafted-chitosan copolymer for ocular delivery of amphotericin B. Int J Nanomed 8:3715–3728
Soliman GM (2017) Nanoparticles as safe and effective delivery systems of antifungal agents: achievements and challenges. Int J Pharm 523(1):15–32
Chaiyasan W, Srinivas SP, Tiyaboonchai W (2015) Crosslinked chitosan-dextran sulfate nanoparticle for improved topical ocular drug delivery. Mol Vis 21:1224–1234
Chen Y-C, Su C-Y, Jhan H-J, Ho H-O, Sheu M-T (2015) Physical characterization and in vivo pharmacokinetic study of self-assembling amphotericin B-loaded lecithin-based mixed polymeric micelles. Int J Nanomed 10:7265–7274
Das S, Suresh PK, Desmukh R (2010) Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine 6(2):318–323
Xu Z, Shi L, Yang M, Zhu L (2019) Preparation and biomedical applications of silk fibroin-nanoparticles composites with enhanced properties—a review. Mater Sci Eng C 95:302–311
Lozano-Perez AA, Rodriguez-Nogales A, Ortiz-Cullera V, Algieri F, Garrido-Mesa J, Zorrilla P, Rodriguez-Cabezas ME, Garrido-Mesa N et al (2014) Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int J Nanomed 9:4507–4520
Pham DT, Saelim N, Tiyaboonchai W (2018) Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: physicochemical properties, crystallinity and structure. J Mater Sci 53(20):14087–14103. https://doi.org/10.1007/s10853-018-2635-3
Sharma S, Bano S, Ghosh AS, Mandal M, Kim H-W, Dey T, Kundu SC (2016) Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomed (NBM) 12(5):1193–1204
Zhang YQ, Ma Y, Xia YY, Shen WD, Mao JP, Zha XM, Shirai K, Kiguchi K (2006) Synthesis of silk fibroin-insulin bioconjugates and their characterization and activities in vivo. J Biomed Mater Res B Appl Biomater 79(2):275–283
Pham DT, Saelim N, Tiyaboonchai W (2018) Design of experiments model for the optimization of silk fibroin based nanoparticles. Int J Appl Pharm 10(5):195–201
Pham DT, Saelim N, Tiyaboonchai W (2019) Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces 181:705–713
Zhao Z, Li Y, Xie MB (2015) Silk fibroin-based nanoparticles for drug delivery. Int J Mol Sci 16(3):4880–4903
Nimtrakul Pataranapa, Tiyaboonchai Waree, Lamlertthon S (2019) Amphotericin B loaded nanostructured lipid carriers for parenteral delivery: characterization, antifungal and in vitro toxicity assessment. Curr Drug Deliv 16(7):645–653
Wong SSW, Kao RYT, Yuen KY, Wang Y, Yang D, Samaranayake LP, Seneviratne CJ (2014) In vitro and in vivo activity of a novel antifungal small molecule against candida infections. PLoS ONE 9(1):e85836
Rodriguez-Tudela JL, Cuenca-Estrella M, Diaz-Guerra TM, Mellado E (2001) Standardization of antifungal susceptibility variables for a semiautomated methodology. J Clin Microbiol 39(7):2513–2517
Takahashi Y, Koike M, Honda H, Ito Y, Sakaguchi H, Suzuki H, Nishiyama N (2008) Development of the short time exposure (STE) test: an in vitro eye irritation test using SIRC cells. Toxicol In Vitro 22(3):760–770
Chanburee S, Tiyaboonchai W (2017) Mucoadhesive nanostructured lipid carriers (NLCs) as potential carriers for improving oral delivery of curcumin. Drug Dev Ind Pharm 43(3):432–440
Niamprem P, Srinivas SP, Tiyaboonchai W (2018) Development and characterization of indomethacin-loaded mucoadhesive nanostructured lipid carriers for topical ocular delivery. Int J Appl Pharm 10(2):91–96
Schuerer N, Stein E, Inic-Kanada A, Pucher M, Hohenadl C, Bintner N, Ghasemian E, Montanaro J et al (2017) Implications for ophthalmic formulations: ocular buffers show varied cytotoxic impact on human corneal-limbal and human conjunctival epithelial cells. Cornea 36(6):712–718
Barwicz J, Christian S, Gruda I (1992) Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob Agents Chemother 36(10):2310–2315
Churchill DN, Seely J (1977) Nephrotoxicity associated with combined gentamicin-amphotericin B therapy. Nephron 19(3):176–181
Adams ML, Kwon GS (2003) Relative aggregation state and hemolytic activity of amphotericin B encapsulated by poly(ethylene oxide)-block–poly(N-hexyl-l-aspartamide)-acyl conjugate micelles: effects of acyl chain length. J Control Release 87(1):23–32
Barwicz J, Tancrède P (1997) The effect of aggregation state of amphotericin-B on its interactions with cholesterol- or ergosterol-containing phosphatidylcholine monolayers. Chem Phys Lipids 85(2):145–155
Zia Q, Khan AA, Swaleha Z, Owais M (2015) Self-assembled amphotericin B-loaded polyglutamic acid nanoparticles: preparation, characterization and in vitro potential against Candida albicans. Int J Nanomed 10:1769–1790
Lemke A, Kiderlen AF, Kayser O (2005) Amphotericin B. Appl Microbiol Biotechnol 68(2):151–162
Torrado JJ, Espada R, Ballesteros MP, Torrado-Santiago S (2008) Amphotericin B formulations and drug targeting. J Pharm Sci 97(7):2405–2425
Merisko-Liversidge E, Liversidge GG, Cooper ER (2003) Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci 18(2):113–120
Dizaj SM, Vazifehasl Z, Salatin S, Adibkia K, Javadzadeh Y (2015) Nanosizing of drugs: effect on dissolution rate. Res Pharm Sci 10(2):95–108
Tiyaboonchai W, Limpeanchob N (2007) Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. Int J Pharm 329(1):142–149
Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ (2002) Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 46:834–840
Hartsel SC, Bauer E, Kwong EH, Wasan KM (2001) The effect of serum albumin on amphotericin B aggregate structure and activity. Pharm Res 18(9):1305–1309
Casa DM, Karam TK, Alves Ade C, Zgoda AA, Khalil NM, Mainardes RM (2015) Bovine serum albumin nanoparticles containing amphotericin B: characterization, cytotoxicity and in vitro antifungal evaluation. J Nanosci Nanotechnol 15(12):10183–10188
Bang JY, Song CE, Kim C, Park WD, Cho KR, Kim PI, Lee SR, Chung WT et al (2008) Cytotoxicity of amphotericin B-incorporated polymeric micelles composed of poly(dl-lactide-co-glycolide)/dextran graft copolymer. Arch Pharm Res 31(11):1463–1469
Acknowledgements
The author would like to thank Miss Manisha Bangar of UCL School of Pharmacy, University College London for her assistance in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interests to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chomchalao, P., Nimtrakul, P., Pham, D.T. et al. Development of amphotericin B-loaded fibroin nanoparticles: a novel approach for topical ocular application. J Mater Sci 55, 5268–5279 (2020). https://doi.org/10.1007/s10853-020-04350-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04350-x