Abstract
Introduction
In the absence of head-to-head trials, this study compared treatment outcomes with the C3 complement inhibitor pegcetacoplan versus the C5 complement inhibitor eculizumab or ravulizumab in complement inhibitor-naïve patients with paroxysmal nocturnal hemoglobinuria (PNH).
Methods
A matching-adjusted indirect comparison was conducted using individual patient data from the pegcetacoplan arm of the PRINCE trial (NCT04085601; n = 34) and aggregate data from the ravulizumab (n = 125) and eculizumab (n = 121) arms of the ALXN1210-PNH-301 trial (NCT03056040). Clinical and quality of life endpoints were evaluated after matching patients in the two trials on baseline characteristics. The weighted Wald test with 95% confidence interval was used to compare categorical and continuous variables (i.e., weighted chi-squared and z tests, respectively). Bias factor analysis was performed to quantify the extent of residual bias from unmeasured confounders.
Results
After weighting, treatment with pegcetacoplan was associated with statistically significant improvements in most clinical endpoints compared with ravulizumab or eculizumab treatment. These included: greater absolute and percent reductions in lactate dehydrogenase (LDH) level and increase in hemoglobin level from baseline; shorter time to first occurrence of LDH normalization; larger proportions of patients achieving hemoglobin stabilization and avoiding transfusion, with fewer packed red blood cell units transfused; and a smaller proportion of patients experiencing breakthrough hemolysis (all p < 0.05). Patients receiving pegcetacoplan also had a greater increase in general health status score from baseline compared with those receiving C5 complement inhibitors.
Conclusion
Pegcetacoplan provides clinical benefits as first-line treatment for complement inhibitor-naïve patients with PNH.
Trial registration
ClinicalTrials.gov identifier, NCT04085601.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Patients with paroxysmal nocturnal hemoglobinuria (PNH) who are treated with ravulizumab and eculizumab may experience persistent symptoms, especially in real-world settings. |
To date, there have been no head-to-head studies comparing pegcetacoplan, a recently approved C3 complement inhibitor, with the C5 complement inhibitors ravulizumab and eculizumab in complement inhibitor-naïve patients with PNH. |
What was learned from the study? |
A matching-adjusted indirect comparison was conducted in order to evaluate the efficacy of pegcetacoplan by comparing individual patient data from the pegcetacoplan arm of the PRINCE trial and aggregate data from the ravulizumab and eculizumab arms of the ALXN1210-PNH-301 trial. |
After weighting by matching patients in the two trials on baseline characteristics, treatment with pegcetacoplan was associated with statistically significant improvements in most clinical endpoints compared with ravulizumab or eculizumab treatment. |
These results suggest that pegcetacoplan has clinical benefits as a first-line treatment for complement inhibitor-naïve patients with PNH. |
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a chronic, rare, debilitating, and life-threatening hemolytic disease characterized by overactivation of the complement system [1,2,3]. The prevalence of PNH in the United States in 2017 was estimated as approximately 13 per 1 million persons, with a median age at diagnosis of 50 years [4, 5]. In the era before complement inhibitors revolutionized the treatment landscape, PNH was associated with premature mortality, with a 10-year survival rate of 76% and median survival of 22 years [6] .
Eculizumab and ravulizumab are C5 complement inhibitors that were approved in 2007 and 2018, respectively [7, 8], for first-line treatment of PNH. Eculizumab treatment has been shown to improve survival rates[9, 10] and result in immediate and sustained improvements in intravascular hemolysis, lactate dehydrogenase (LDH) level, anemia, thromboembolic events, transfusion avoidance, and quality of life (QoL) measures, such as fatigue and global health status [2, 3, 11,12,13,14]. Low mortality rates were also observed with ravulizumab in clinical trials [15], and ravulizumab was shown to be noninferior to eculizumab with respect to all endpoints examined in the phase 3 ALXN1210-PNH-301 trial (NCT02946463) [16]. However, despite their efficacy, many patients with PNH who receive ravulizumab or eculizumab have persistent symptoms, especially in real-world settings [1, 17]. In one retrospective study, 72% of patients with PNH who received eculizumab for at least 13 months remained anemic and 36% required at least one transfusion per year [18]. It was also reported that 25%–35% of patients continue to require transfusions following eculizumab treatment [1, 19].
Pegcetacoplan is a C3 complement inhibitor that was recently approved by the US Food and Drug Administration for the treatment of complement inhibitor-naïve adult patients with PNH and complement inhibitor-experienced patients switching from eculizumab or ravulizumab [20, 21]. As C3 acts upstream of C5 in the complement cascade, its inhibition has a broader effect than C5 inhibition; thus, pegcetacoplan mitigates hemolysis more effectively than C5 inhibitors, resulting in better outcomes in patients with PNH [22]. In the phase 3 PRINCE trial (NCT04085601), meaningful clinical and hematologic improvements were observed in complement inhibitor-naïve patients with PNH who were treated with pegcetacoplan [23].
To date, there have been no head-to-head studies comparing pegcetacoplan with ravulizumab or eculizumab in complement inhibitor-naïve patients with PNH. However, in the phase 3 PEGASUS trial (NCT03500549), pegcetacoplan was superior to eculizumab in improving hemoglobin levels from baseline in patients who had previously received eculizumab, and was noninferior in terms of decreasing the reticulocyte count from baseline and the proportion of patients who remained transfusion-free during the study [22]. In the absence of a direct comparison, a matching-adjusted indirect comparison (MAIC) was conducted using data from the PEGASUS trial and the phase 3 ALXN1210-PNH-302 trial (NCT03056040) of ravulizumab versus eculizumab in patients previously treated with eculizumab [24, 25]. By matching baseline characteristics using individual patient data (IPD) from PEGASUS and aggregate data from ALXN1210-PNH-302, it was demonstrated that treatment with pegcetacoplan resulted in hemoglobin stabilization and LDH normalization, alleviated the need for transfusion as well as transfusion requirements, and improved intravascular hemolysis and QoL outcomes compared with ravulizumab treatment.
In order to assess the relative effectiveness of pegcetacoplan versus C5 complement inhibitors, the present study used the MAIC approach to compare treatment outcomes in complement inhibitor-naïve patients with PNH, using IPD from the pegcetacoplan arm of the PRINCE trial and aggregate data from the ravulizumab and eculizumab arms of the ALXN1210-PNH-301 trial [26].
Methods
Compliance with Ethics Guidelines
The PRINCE[27] and ALXN1210-PNH-301[28] trials were conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The study protocols were approved by the institutional review board or ethics committee at each study site, and all patients provided written informed consent.
Data Sources
PRINCE was a phase 3, multicenter, randomized, controlled, open-label study of pegcetacoplan in complement inhibitor-naïve patients with PNH who had not received treatment with any complement inhibitor (e.g., eculizumab) in the 3 months prior to screening. The study period was 08/2019–06/2021 [27]. A total of 53 patients were randomized to receive either pegcetacoplan (n = 35) or supportive care (n = 18) for 26 weeks during the randomized controlled period. ALXN1210-PNH-301 was a phase 3, multicenter, randomized, active-controlled, open-label study comparing ravulizumab and eculizumab in complement inhibitor-naïve patients with PNH. The study started in 12/2016 and is estimated to be completed in 2023 [28]. A total of 246 patients were randomized to receive ravulizumab (n = 125) or eculizumab (n = 121) for 26 weeks. Aggregate data from ALXN1210-PNH-301 were sourced from a published report[16] except for hemoglobin levels, which were extracted from a separate abstract[29] using WebPlotDigitizer (Version 4.5).
The patients included in the MAIC were ≥ 18 years old, were naïve to complement inhibitor treatment, had received meningococcal vaccination, had an absolute neutrophil count > 500/mm3 , and an adequate platelet count (> 50,000/mm3 in the PRINCE study and > 30,000/mm3 in the ALXN1210-PNH-301 study) at the screening visit, and had no previous history of bone marrow transplantation. The PRINCE study excluded patients who had received treatment with any complement inhibitor within 3 months prior to screening, whereas the ALXN1210-PNH-301 study excluded patients with any current or previous exposure to complement inhibitor.
Study Endpoints
Endpoints were similarly defined in the PRINCE and ALXN1210-PNH-301 studies (Table 1). Clinical endpoints included: absolute and percent changes in LDH level from baseline, rate of LDH normalization, and time to first occurrence of LDH normalization; absolute and percent changes in hemoglobin level from baseline and rate of hemoglobin stabilization; rate of transfusion avoidance and transfusion requirements [as the total number of packed red blood cell (PRBC) units transfused]; rate of breakthrough hemolysis; and rate of major adverse vascular events (MAVEs). When calculating the time to first occurrence of LDH normalization, patients were not required to maintain normal LDH levels after the first occurrence according to the ALXN1210-PNH-301 trial definition [16]. QoL endpoints included absolute change from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score and absolute change from baseline in European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) scores (for general health status, physical functioning, and fatigue symptoms).
Statistical Methods
To estimate the likelihood of enrollment in the ALXN1210-PNH-301 study versus in the PRINCE study, a propensity score model based on logistic regression was used to assign weights to each patient in the PRINCE IPD. Matching was performed such that the weighted means and proportions of baseline characteristics in the PRINCE study IPD matched those of the ALXN1210-PNH-301 study aggregate data. The weight applied to each patient in the PRINCE IPD was equal to the inverse odds of their enrollment in the ALXN1210-PNH-301 study versus in the PRINCE study. Separate sets of weights were generated to compare pegcetacoplan to ravulizumab and pegcetacoplan to eculizumab. Model adequacy was assessed by considering effective sample size (ESS) and through visual inspection of histograms of patient weights. Adequate models were required to have an ESS of at least 50% of the initial PRINCE study population. Because of sample size limitations, it was not possible to adjust for all effect modifiers. Patients from the PRINCE study were weighted on Asian race, age at first infusion, female sex, and baseline EORTC general health score.
Before matching, the Wald test with 95% confidence interval (CI) was used to compare categorical and continuous variables (i.e., chi-squared and z tests, respectively). After matching, outcomes were compared between balanced treatment groups using statistical tests that incorporated weights generated during matching. The weighted Wald test with 95% CI was used for comparisons of categorical and continuous variables (i.e., weighted chi-squared and z tests, respectively).
A bias factor analysis was conducted to quantify the extent of residual bias from unmeasured confounders, which provided a set of adjusted results of the unanchored MAIC. A set of potential confounders that were binary baseline variables (e.g., age ≥ 65 years, overweight/obese, history of aplastic anemia) was selected, and a bias factor was calculated for each. Unanchored indirect comparisons were separately adjusted for each bias factor by subtracting the factor from the effect estimate and 95% CI. Additional details and results of the bias factor analysis can be found in the Supplementary Materials.
Results
Of the 35 patients from the pegcetacoplan arm of the PRINCE study, 34 were included in the current analysis, whereas one was excluded because of a lack of LDH and hemoglobin data after baseline. After weights were applied to match baseline characteristics of these patients to those of patients in the ravulizumab and eculizumab arms of the ALXN1210-PNH-301 study, the effective sample sizes of the pegcetacoplan arm were 24 and 22, matched to 125 patients from the ravulizumab arm and 121 from the eculizumab arm, respectively.
Baseline Characteristics
Before weighting, there were significant differences between the pegcetacoplan and ravulizumab arms in the following baseline characteristics: White race, American Indian or Alaska Native race, mean LDH level, and EORTC QLQ-C30 general health score. Except for the EORTC QLQ-C30 general health score, these characteristics also differed between the pegcetacoplan and eculizumab arms at baseline (Table 2).
After separately weighting the pegcetacoplan arm (on Asian race, age at first infusion, female sex, and EORTC QLQ-C30 general health score) to match the ravulizumab and eculizumab arms, there was a larger proportion of patients who were American Indian or Alaska Native in the pegcetacoplan arm than in the ravulizumab (30.4% vs. 0.8%, p = 0.0026) or eculizumab (36.7% vs. 0.8%, p = 0.0008) arms. Mean baseline LDH level was also higher in patients who received pegcetacoplan compared with ravulizumab (2,220.27 U/l vs. 1,633.50 U/l, p = 0.0004) or eculizumab (2,291.04 U/l vs. 1,578.30 U/l, p < 0.0001). No other baseline characteristics differed significantly between patients treated with pegcetacoplan versus with ravulizumab or eculizumab after weighting (Table 3).
Clinical and Hematologic Endpoints
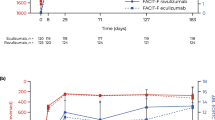
After weighting, treatment with pegcetacoplan was associated with statistically significant improvements in most clinical and hematologic endpoints compared with ravulizumab or eculizumab treatment (Figs. 1, 2, 3, 4). This included greater absolute and percent reductions in LDH level from baseline compared with ravulizumab (− 764.05 U/l and − 11.89%, respectively, both p < 0.0001) (Fig. 1) and eculizumab (− 886.85 U/l and − 12.42%, respectively, both p < 0.0001) (Fig. 2). The proportion of patients who achieved LDH normalization was larger with pegcetacoplan than with ravulizumab (25.95%, p = 0.0093) or eculizumab (26.56%, p = 0.0154). Additionally, patients who received pegcetacoplan had a shorter time to first occurrence of LDH normalization than those who received ravulizumab (− 8.29 days, p < 0.0001) or eculizumab (− 13.07 days, p = 0.0095).
Unanchored comparisons between pegcetacoplan and ravulizumab – hematologic endpoints. 1. The following baseline characteristics were used for weighting: Asian race, age at first infusion, female sex, and baseline EORTC general health score. 2. Change in hemoglobin level in the ALXN1210-PNH-301 study was estimated from values for percent hemoglobin stabilization and mean hemoglobin levels reported by Lee et al. [16] and Schrezenmeier et al. [29]. *Significant p values
Unanchored comparisons between pegcetacoplan and eculizumab – hematologic endpoints. 1. The following baseline characteristics were used for weighting: Asian race, age at first infusion, female sex, and baseline EORTC general health score. 2. Change in hemoglobin in the ALXN1210-PNH-301 study was estimated from values for percent hemoglobin stabilization and mean hemoglobin levels reported by Lee et al. [16] and Schrezenmeier et al. [29]. *Significant p values
After weighting, pegcetacoplan was also associated with greater absolute and percent increases in hemoglobin levels from baseline compared with ravulizumab (1.58 g/dl, p = 0.0193 and 16.84%, p = 0.0223, respectively; Fig. 3) and eculizumab (1.78 g/dl, p = 0.0289 and 19.49%, p = 0.0291, respectively; Fig. 4). A larger proportion of patients treated with pegcetacoplan achieved hemoglobin stabilization than patients who received ravulizumab (26.35%, p < 0.0001) or eculizumab (27.73%, p = 0.0001). After weighting, more patients who received pegcetacoplan avoided transfusion during the randomized controlled period than patients who received ravulizumab (20.75%, p = 0.0003) or eculizumab (26.13%, p = 0.0002). Pegcetacoplan treatment was also associated with fewer PRBC units transfused than ravulizumab (− 4.07 units, p < 0.0001) or eculizumab (− 4.62 units, p < 0.0001).
No patients in the pegcetacoplan arm experienced breakthrough hemolysis or MAVEs. After weighting, a smaller proportion of patients experienced breakthrough hemolysis when treated with pegcetacoplan versus with ravulizumab (− 4.00%, p = 0.0225; Fig. 5) or eculizumab (− 10.70%, p = 0.0001; Fig. 6). However, there was no significant difference in the proportion of patients who experienced MAVEs with pegcetacoplan versus with ravulizumab (p = 0.1540) or eculizumab (p = 0.3153).
Unanchored comparisons between pegcetacoplan and ravulizumab – safety and QoL endpoints. 1. The following baseline characteristics were used for weighting: Asian race, age at first infusion, female sex, and baseline EORTC general health score. 2. Breakthrough hemolysis was defined as ≥ 1 new or worsening sign or symptom of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin < 10 g/dl], or MAVEs) in the presence of LDH ≥ 2 × ULN after prior reduction to < 1.5 × ULN with treatment. 3. MAVEs were defined as: thrombophlebitis/deep vein thrombosis; pulmonary embolus; myocardial infarction; transient ischemic attack; unstable angina; renal vein thrombosis; acute peripheral vascular occlusion; mesenteric/visceral vein thrombosis or infarction; mesenteric/visceral arterial thrombosis or infarction; hepatic/portal vein thrombosis (Budd–Chiari syndrome); cerebral arterial occlusion/cerebrovascular accident; cerebral venous occlusion; renal arterial thrombosis; gangrene (non-traumatic; nondiabetic); amputation (non-traumatic; nondiabetic); and dermal thrombosis. *Significant p values
Unanchored comparisons between pegcetacoplan and eculizumab -- safety and QoL endpoints. 1. The following baseline characteristics were used for weighting: Asian race, age at first infusion, female sex, and baseline EORTC general health score. 2. Breakthrough hemolysis was defined as ≥ 1 new or worsening sign or symptom of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin < 10 g/dl], or MAVEs) in the presence of LDH ≥ 2 × ULN after prior reduction to < 1.5 × ULN with treatment. 3. MAVEs were defined as: thrombophlebitis/deep vein thrombosis; pulmonary embolus; myocardial infarction; transient ischemic attack; unstable angina; renal vein thrombosis; acute peripheral vascular occlusion; mesenteric/visceral vein thrombosis or infarction; mesenteric/visceral arterial thrombosis or infarction; hepatic/portal vein thrombosis (Budd–Chiari syndrome); cerebral arterial occlusion/cerebrovascular accident; cerebral venous occlusion; renal arterial thrombosis; gangrene (non-traumatic; nondiabetic); amputation (non-traumatic; nondiabetic); and dermal thrombosis. *Significant p values
QoL Endpoints
Pegcetacoplan was associated with greater increases in EORTC QLQ-C30 general health status score from baseline than ravulizumab (12.91, p = 0.0087; Fig. 5) and eculizumab (12.52, p = 0.0133; Fig. 6). For the other QoL outcomes, there were no significant differences between pegcetacoplan and ravulizumab or eculizumab treatment. Changes in FACIT-Fatigue score, EORTC QLQ-C30 physical functioning score, and EORTC QLQ-C30 fatigue symptoms score were comparable with pegcetacoplan versus with ravulizumab (p = 0.2973, 0.0682, and 0.4870, respectively) and with pegcetacoplan versus with eculizumab (p = 0.1667, 0.2218, and 0.2860, respectively).
Discussion
The present study is the first to use the MAIC approach to compare the effectiveness of pegcetacoplan versus ravulizumab and eculizumab in complement inhibitor-naïve patients with PNH. In the unanchored comparisons of clinical, hematologic, and QoL endpoints, treatment with pegcetacoplan was associated with favorable outcomes and a suggestion of improvement in all measured laboratory parameters compared with ravulizumab and eculizumab.
LDH is a marker of intravascular hemolysis, and elevated LDH levels are associated with an increased risk of life-threatening complications such as thromboembolism in patients with PNH, making LDH level an important indicator of disease severity [12, 14, 30]. As such, past studies assessing treatments for PNH have included LDH-based outcomes as primary efficacy endpoints [16, 25, 31]. The results of the present study show that patients receiving pegcetacoplan had significantly greater reductions in LDH level than those receiving ravulizumab or eculizumab; they were also more likely to achieve LDH normalization, and achieved it more rapidly. LDH was also used in the PRINCE and ALXN1210-PNH-301 studies as a marker of breakthrough hemolysis. This condition is broadly characterized by intravascular hemolysis and the reappearance of classical PNH symptoms in patients undergoing treatment for PNH, and may be linked to suboptimal C5 inhibition and/or higher C3b density resulting from complement-amplifying conditions (e.g., infection, surgery, or pregnancy) [32]. The reduction in breakthrough hemolysis in patients receiving pegcetacoplan may thus reflect more effective inhibition of C5 through targeting of C3, in addition to direct inhibition of C3.
Hemoglobin level and stabilization are clinical indicators of disease severity in hemolytic diseases such as PNH, and have been used to define categories of response (e.g., optimal or partial) to treatment [33, 34]. In this study, patients receiving pegcetacoplan had significantly greater increases in both absolute and percent hemoglobin levels from baseline and a larger proportion achieved hemoglobin stabilization compared with patients receiving ravulizumab or eculizumab. Accordingly, transfusion requirement was lower and the rate of transfusion avoidance higher in the pegcetacoplan arm. These results are consistent with findings from another MAIC of pegcetacoplan and ravulizumab in patients previously treated with eculizumab [24].
PNH and its symptoms have negative effects on patients’ QoL [35,36,37,38]. Of the four QoL measures used as endpoints in the studies compared in the present analysis, only the EORTC QLQ-C30 global health status score was significantly improved by pegcetacoplan. This contrasts with a previous MAIC showing that all four QoL measures were improved with pegcetacoplan in patients previously treated with eculizumab [24]. The discrepancy may be explained by the difference in QoL scores between the ALXN1210-PNH-301 and ALXN1210-PNH-302 studies: scores for both the ravulizumab and eculizumab arms were higher in the former [16, 25]. Meanwhile, as scores for the pegcetacoplan arms were similar between the PRINCE and PEGASUS studies, the relative improvement seen in patients from the ALXN1210-PNH-301 study may have diminished the gap that was observed in the previous MAIC. Although the objective of the present study was not to examine adverse events or toxicity, it should be noted that in the PEGASUS study, injection site reactions and diarrhea were more frequently observed with pegcetacoplan than what was reported in the ALXN1210-PNH-301 study with eculizumab (37% vs. 3% and 22% vs. 3%, respectively) [22]. The former was likely related to the route of administration of pegcetacoplan (subcutaneous) compared with eculizumab (intravenous), as injection site reactions were also found to be more common with subcutaneous versus intravenous ravulizumab [7]. On the other hand, headache has been reported in a substantial proportion of patients receiving C5 inhibitors (23–44%, compared with 7% of patients in the pegcetacoplan arm of the PEGASUS study) [7, 8, 21]. Additional studies are needed in order to determine the degree to which these various adverse events impacts the QoL of patients with PNH.
Although an economic analysis of C3 versus C5 inhibitors was beyond the scope of this work, the relative costs of these treatments also warrant examination in the future, as this topic is of considerable interest to healthcare stakeholders. A cost-effectiveness analysis of patients with PNH in the United States (US) showed that ravulizumab provided cost savings of US$1,000,818 per quality-adjusted life-year gained compared with eculizumab [39]. Another study estimated that for a US health plan with 10 million members, including pegcetacoplan as a treatment option for PNH resulted in cost savings of $1.2–$2.2 million that were attributable to lower costs of drug acquisition and administration, reduced requirement for blood transfusion, and decreased rates of breakthrough hemolysis [40]. Moreover, higher response rates were achieved at a lower cost with pegcetacoplan than with eculizumab [41]. Pegcetacoplan was also associated with lower healthcare costs compared with ravulizumab in the United Kingdom [42]. These results suggest that pegcetacoplan has economic as well as clinical benefits.
The interpretation of this study’s results is subject to limitations. MAICs only account for cross-trial differences that are observable in the data [26]. The comparator trial was selected with the aim of minimizing cross-trial differences in design and conduct, but there were still differences between the two studies that could not be adjusted for by statistical analyses (e.g., route of administration and treatment administration schedule). For example, the ALXN1210-PNH-301 study excluded patients with any current or previous exposure to complement inhibitor, whereas the PRINCE study excluded only those who received treatment with a complement inhibitor in the 3 months before the screening visit. Additionally, although matching was applied to observed cross-trial differences in patient characteristics to the extent possible, there may have been unobserved differences that influenced the results. Because of differences across studies, not all potential effect modifiers could be used as weighting variables. As an unanchored MAIC, it was assumed that absolute treatment effects were constant at any given level of effect modifiers and prognostic variables, which were themselves accounted for. As it was difficult to guarantee this assumption, a bias factor analysis was conducted to estimate the effects of selected unmeasured confounders (see Supplementary Materials). Like all MAICs, the validity of this study relied on the implicit assumption of the internal validity of the studies being compared (i.e., the PRINCE and ALXN1210-PNH-301 studies). Lastly, the IPD of the PRINCE study were weighted to match the aggregate data of the ALXN1210-PNH-301 study for the purpose of comparison; therefore, the results may not be fully generalizable to other PNH patient populations.
Conclusions
The results of this study suggest that pegcetacoplan provides clinical benefits as first-line treatment for complement inhibitor-naïve patients with PNH. In the absence of head-to-head trials, a MAIC approach allowed a comparison of clinical effectiveness between pegcetacoplan and C5 complement inhibitors. Patients treated with pegcetacoplan had greater improvements in clinical, hematologic, and QoL outcomes than those treated with either ravulizumab or eculizumab in this analysis. These findings can potentially guide clinical decision-making for optimized treatment of patients with PNH.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available. Data used for this study are from Apellis Pharmaceuticals, Inc. and were used under license for the current study. Therefore, restrictions apply to the availability of these data, which cannot be made publicly available. Access to this dataset may be granted to other interested parties by Apellis Pharmaceuticals, Inc, but may be subject to limitations based on how the original clinical trial data were acquired.
Change history
04 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12325-023-02640-9
References
Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–11.
Hillmen P, Elebute M, Kelly R, Urbano-Ispizua A, Hill A, Rother RP, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85(8):553–9.
Hillmen P, Muus P, Roth A, Elebute MO, Risitano AM, Schrezenmeier H, et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162(1):62–73.
Jalbert JJ, Chaudhari U, Zhang H, Weyne J, Shammo JM. Epidemiology of PNH and Real-World Treatment Patterns Following an Incident PNH Diagnosis in the US. Blood. 2019;134:3407.
Shah N, Bhatt H. Paroxysmal nocturnal hemoglobinuria. Treasure Island: StatPearls; 2022.
de Latour RP, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. J Am Soc Hematol. 2008;112(8):3099–106.
US Food and Drug Administration. ULTOMIRIS (ravulizumab-cwvz) Prescribing information.. Alexion Pharmaceuticals: Boston, MA. 2022. https://alexion.com/documents/ultomiris_uspi. Accessed 03 Jan 2023.
US Food and Drug Administration. SOLIRIS (eculizumab) Prescribing Information.. Alexion Pharmaceuticals: Boston, MA. 2020. https://alexion.com/Documents/Soliris_USPI.pdf. Accessed 03 Jan 2023.
Loschi M, Porcher R, Barraco F, Terriou L, Mohty M, de Guibert S, et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: a treatment versus no-treatment study. Am J Hematol. 2016;91(4):366–70.
Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. J Am Soc Hematol. 2011;117(25):6786–92.
Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111(4):1840–7.
Hill A, Hillmen P, Richards SJ, Elebute D, Marsh JC, Chan J, et al. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005;106(7):2559–65.
Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–43.
Hillmen P, Muus P, Duhrsen U, Risitano AM, Schubert J, Luzzatto L, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–8.
Kulasekararaj A, Brodsky R, Griffin M, Kulagin A, Ogawa M, Wang J, et al. P812: Long-term complement inhibition and survival outcomes in patients with paroxysmal nocturnal hemoglobinuria: An interim analysis of the ravulizumab clinical trials. HemaSphere. 2022;6:706–7.
Lee JW, Sicre de Fontbrune F, Wong LLL, Pessoa V, Gualandro S, Füreder W, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–9.
DeZern AE, Dorr D, Brodsky RA. Predictors of hemoglobin response to eculizumab therapy in paroxysmal nocturnal hemoglobinuria. Eur J Haematol. 2013;90(1):16–24.
McKinley CE, Richards SJ, Munir T, Griffin M, Mitchell LD, Arnold L, et al. Extravascular hemolysis due to C3-loading in patients with PNH treated with eculizumab: defining the clinical syndrome. Blood. 2017;130(1):3471.
Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, Xhaard A, Rosain J, Castaneda DC, et al. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2015;125(5):775–83.
US Food and Drug Administration. FDA approves new treatment for adults with serious rare blood disease. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-new-treatment-adults-serious-rare-blood-disease.
US Food and Drug Administration. EMPAVELI (pegcetacoplan) Prescribing Information. Apellis Pharmaceuticals: Waltham, MA. 2021. https://pi.apellis.com/files/PI_Empaveli.pdf. Accessed 03 Jan 2023.
Hillmen P, Szer J, Weitz I, Roth A, Hochsmann B, Panse J, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2021;384(11):1028–37.
Wong RS, Navarro JR, Comia NS, Goh YT, Idrobo H, Kongkabpan D, et al. Efficacy and safety of pegcetacoplan treatment in complement-inhibitor naïve patients with paroxysmal nocturnal hemoglobinuria: results from the phase 3 Prince study. Blood. 2021;138:606.
Bhak RH, Mody-Patel N, Baver SB, Kunzweiler C, Yee CW, Sundaresan S, et al. Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: a matching-adjusted indirect comparison. Curr Med Res Opin. 2021;37(11):1913–23.
Kulasekararaj AG, Hill A, Rottinghaus ST, Langemeijer S, Wells R, Gonzalez-Fernandez FA, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540–9.
Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value in Health. 2012;15(6):940–7.
Clinicaltrials.gov. A study to evaluate the efficacy and safety of pegcetacoplan in patients with PNH. ClinicalTrials.gov identifier: NCT04085601. 2022. Updated 21 Oct 2022. https://clinicaltrials.gov/ct2/show/NCT04085601. Accessed 03 Jan 2022.
Clinicaltrials.gov. ALXN1210 (ravulizumab) versus eculizumab in complement inhibitor treatment-naïve adult participants with paroxysmal nocturnal hemoglobinuria (PNH). ClinicalTrials.gov identifier: NCT02946463. 2022. Updated 09 Aug 2022. https://clinicaltrials.gov/ct2/show/NCT02946463. Accessed 03 Jan 2022.
Schrezenmeier H, Kulasekararaj AG, Mitchell L, Peffault De Latour R, Devos T, Okamoto S, et al. Predictors for improvement in patient-reported outcomes: post-hoc analysis of a phase 3 randomized, open-label study of eculizumab and ravulizumab in complement inhibitor-naïve patients with paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2021;138(1):2196.
Lee JW, Jang JH, Kim JS, Yoon SS, Lee JH, Kim YK, et al. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013;97(6):749–57.
Roth A, Rottinghaus ST, Hill A, Bachman ES, Kim JS, Schrezenmeier H, et al. Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv. 2018;2(17):2176–85.
Brodsky RA, Peffault de Latour R, Rottinghaus ST, Roth A, Risitano AM, Weitz IC, et al. Characterization of breakthrough hemolysis events observed in the phase 3 randomized studies of ravulizumab versus eculizumab in adults with paroxysmal nocturnal hemoglobinuria. Haematologica. 2021;106(1):230–7.
Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers. 2015;2015: 635670.
Risitano AM, Marotta S, Ricci P, Marano L, Frieri C, Cacace F, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157.
Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manag. 2002;24(6):547–61.
Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–38.
Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, et al. EORTC QLQ-C30 reference values. Brussels: EORTC Quality of Life Group; 2008.
Snyder CF, Blackford AL, Sussman J, Bainbridge D, Howell D, Seow HY, et al. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients’ supportive care needs. Qual Life Res. 2015;24(5):1207–16.
O’Connell T, Buessing M, Johnson S, Tu L, Thomas SK, Tomazos I. Cost-utility analysis of ravulizumab compared with eculizumab in adult patients with paroxysmal nocturnal hemoglobinuria. Pharmacoeconomics. 2020;38(9):981–94.
Anderson S, Talbird SE, Fishman J, Mody-Patel N, Sarda SP. POSB145 budget impact of pegcetacoplan, a complement c3 inhibitor, for the treatment of paroxysmal nocturnal hemoglobinuria in US adults. Value in Health. 2022;25(1):S89.
Anderson S, Talbird S, Fishman J. Cost per responder analysis for pegcetacoplan and eculizumab in the treatment of adults with paroxysmal nocturnal hemoglobinuria. Blood. 2021;138(1):4956.
Hakimi Z, Wilson K, McAughey E, Pochopien M, Wojciechowski P, Toumi M, et al. The cost-effectiveness, of pegcetacoplan compared with ravulizumab for the treatment of paroxysmal nocturnal hemoglobinuria, in a UK setting. J Comp Eff Res. 2022;11(13):969–85.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Janice Imai, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Apellis Pharmaceuticals, Inc.
Authorship
All authors agree to be accountable for all aspects of the work and read and approved the final manuscript.
Prior Presentation
Part of the material in this manuscript was presented as posters at the European Hematology Association 2022 Congress (June 9–12, 2022 in Vienna, Austria) and 10th Annual Meeting of the Society of Hematologic Oncology 2022 (September 28–October 1, 2022 in Houston, Texas, USA); and as an abstract at the 64th American Society for Hematology Annual Meeting and Exposition (December 10–13, 2022 in New Orleans, Louisiana, USA).
Funding
This research, the Rapid Service Fee, and the Open Access fee were sponsored by Apellis Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study; drafting and critical revision of the article for important intellectual content; and approved the final version. All authors agree to be accountable for all aspects of this study.
Corresponding author
Ethics declarations
Disclosures
Raymond Wong was an investigator on the PRINCE trial funded by Apellis Pharmaceuticals. Jesse Fishman, Michael Yeh, and Mohammed Al-Adhami are employees of Apellis Pharmaceuticals, Inc. and own stock/stock options. Koo Wilson is an employee of Sobi AB and holds company shares. Abigail Zion, Christopher W. Yee, Lynn Huynh, and Mei Sheng Duh are employees of Analysis Group Inc., which received research funding from Apellis Pharmaceuticals, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers of this manuscript have no relevant financial or other relationships to disclose.
Compliance with Ethics Guidelines
The two trials that were the basis of the analyses performed in this study were conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The study protocols were approved by the institutional review board or ethics committee at each study site, and all patients provided written informed consent.
Additional information
This article was revised due to error in Tables 2,3 and figure 2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wong, R., Fishman, J., Wilson, K. et al. Comparative Effectiveness of Pegcetacoplan Versus Ravulizumab and Eculizumab in Complement Inhibitor-Naïve Patients with Paroxysmal Nocturnal Hemoglobinuria: A Matching-Adjusted Indirect Comparison. Adv Ther 40, 1571–1589 (2023). https://doi.org/10.1007/s12325-023-02438-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02438-9