Abstract
Introduction
The healthcare resource utilization (HRU) and costs of oral anticoagulant-naïve patients with non-valvular atrial fibrillation (NVAF) and diabetes initiated on rivaroxaban or warfarin in the United States (US) has not been previously evaluated.
Methods
This retrospective study used data from the Optum’s de-identified Clinformatics® Data Mart Database (1 January, 2012 to 30 September, 2021) to evaluate the HRU and costs of adult patients with NVAF and diabetes newly initiated on rivaroxaban or warfarin (on or after January 2013). Inverse probability of treatment weighting (IPTW) was used to adjust for confounding between cohorts. HRU and costs (USD 2021) were assessed per patient-year (PPY) post-treatment initiation. Weighted cohorts were compared using rate ratios (RR) from Poisson regression models, odds ratios (OR) from logistic regression models, and cost differences; 95% confidence intervals (CI) and p values were generated using non-parametric bootstrap procedures.
Results
After IPTW, 17,881 and 19,274 patients initiated on rivaroxaban and warfarin were included, respectively (mean age: 73 years; 40% female). During 12 months of follow-up, the rivaroxaban cohort had lower all-cause HRU PPY across all components, including lower rates of inpatient stays (RR: 0.84, 95% CI 0.81, 0.88), outpatient visits (RR: 0.67, 95% CI 0.66, 0.68), and 30 day hospital readmission (OR: 0.75, 95% CI 0.66, 0.83; all p < 0.001) compared to the warfarin cohort. Moreover, rivaroxaban was associated with medical cost savings PPY (mean cost difference: − $9306, 95% CI − $11,769, − $6607), which compensated for higher pharmacy costs relative to warfarin (mean cost difference: $5518, 95% CI $5193, $5839), resulting in significantly lower all-cause total healthcare costs for rivaroxaban versus warfarin (mean cost difference: − $3788, 95% CI − $6258, − $1035; all p < 0.001).
Conclusion
Among NVAF patients with diabetes in a real-world US setting, rivaroxaban was associated with lower healthcare costs compared to warfarin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Despite diabetes being increasingly prevalent in the US, the scientific literature is scarce on the impact of rivaroxaban and warfarin in patients with both NVAF and diabetes. |
This study compared the healthcare resource utilization and costs of patients with NVAF and diabetes treated with rivaroxaban versus warfarin. |
What was learned from this study? |
Patients with NVAF and diabetes treated with rivaroxaban had significantly less hospitalizations and outpatient visits, and significantly lower healthcare costs compared to patients treated with warfarin. |
Rivaroxaban may play an important role in reducing the economic burden associated with NVAF and diabetes in the US. |
Introduction
Diabetes is commonly prevalent in patients with atrial fibrillation (AF) [1,2,3], and is associated with a 23–49% greater risk of developing AF [4]. Evidence further suggests that the presence of comorbid diabetes among patients with AF may lead to worse clinical outcomes than either condition alone [5,6,7,8]. The results of large, nationwide registry studies indicate that AF patients with diabetes have significantly higher rates of hospitalization, cardiovascular mortality, and overall mortality, as well as worse symptoms and poorer quality of life compared to AF patients without diabetes [6, 7]. Furthermore, a study using Nationwide Inpatient Sample inpatient data from 2004 to 2014 observed a significant 4.4% increase in the rate of AF-related hospitalizations among AF patients with comorbid diabetes over time compared to those without [7].
Anticoagulation therapy is the cornerstone treatment for non-valvular AF (NVAF) [9,10,11], the most common type of AF in the US (US), which accounts for over 15% of all strokes [12]. Vitamin K antagonists (VKAs; e.g., warfarin) were the first agents used to reduce the risk of stroke and mortality among NVAF patients. However, notable disadvantages of VKAs are that they require regular monitoring with dosage adjustments, and are associated with numerous drug and food interactions [8, 13, 14]. In the past decade, non-vitamin K direct oral anticoagulants (DOACs; e.g., rivaroxaban) have been approved by the US Food and Drug Administration for the treatment of NVAF [15,16,17]. Several randomized clinical trials have demonstrated that DOACs such as rivaroxaban are at least as effective as VKAs for stroke prevention in patients with NVAF [18,19,20,21,22], while maintaining a similar, if not improved, safety profile to that of VKAs without the need for laboratory monitoring. Per the 2020 European Society of Cardiology guidelines [8], DOACs such as rivaroxaban may also be considered a viable alternative to warfarin among NVAF patients with diabetes based on supporting evidence from clinical trials [23, 24]. For instance, in secondary analyses of the ROCKET AF clinical trial data, the relative efficacy and safety of rivaroxaban were similar in NVAF patients with and without diabetes when compared to warfarin [21, 25]. More recently, retrospective claims-based studies have suggested that rivaroxaban may have a favorable effectiveness and/or safety profile compared to warfarin among NVAF patients with diabetes in real-world clinical practice in the US [26, 27].
The economic burden associated with NVAF in the US is substantial, with annual NVAF-related costs estimated at $6 billion, and rising to $26 billion when other cardiovascular and non-cardiovascular costs are included [28]. Current data suggest that anticoagulant therapy with rivaroxaban may reduce these healthcare costs among patients with NVAF when compared to warfarin [29,30,31,32]. Further, rivaroxaban has been associated with lower healthcare costs compared to warfarin among complex patients with comorbid conditions such as obesity [33,34,35], as well as those with concurrent NVAF, obesity, and diabetes [36]. Based on this evidence, it is plausible that rivaroxaban might confer economic benefits among patients with NVAF and diabetes, irrespective of body weight. However, no study to date has specifically examined the economic impact of anticoagulation therapy in this broader population of NVAF patients with diabetes. Such inquiries would be an important addition to the literature, as diabetes is becoming increasingly prevalent in the US, even among individuals of normal body weight [37,38,39].
Accordingly, this study compared the healthcare resource utilization (HRU) and healthcare costs associated with rivaroxaban and warfarin among oral anticoagulant-naïve patients with NVAF and diabetes, thereby addressing this knowledge gap regarding the economic benefits of treatment and informing future therapeutic decisions.
Methods
Data Source
This study used data from the Optum’s de-identified Clinformatics® Data Mart Database (CDM) spanning from January 1, 2012, to September 30, 2021. CDM is a large de-identified administrative claims database containing healthcare claims, which covers approximately 15–20 million annual lives for a total of roughly 62 million unique lives in all US census regions. This database comprises both commercial and Medicare Advantage health plans, and includes data on patient demographics, dates of eligibility, date of death (sourced from the Social Security Administration’s Death Master File), claims for inpatient (IP) and outpatient (OP) visits, pharmacy encounters, and costs of services [40]. Data were de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101). Furthermore, no consent to participate or for publication was required. Finally, this study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Study Design and Population
A retrospective weighted-cohort design was used to evaluate and compare outcomes among patients with NVAF and diabetes who were newly initiated on rivaroxaban or warfarin. Figure 1 presents the study design scheme. Patients eligible for the study were required to meet the following inclusion criteria: (1) ≥ 1 dispensing for rivaroxaban or warfarin, where the date of the first dispensing was defined as the index date; (2) ≥ 12 months of continuous health plan enrollment before the index date, defined as the baseline period; (3) ≥ 1 diagnosis of AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 427.31, or International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes: I48.0x–148.2x, 148.91) during the baseline period or on the index date; (4) diabetes at baseline, identified as ≥ 2 diagnoses of diabetes (including type 1 and 2; ICD-9-CM codes: 250.x, and ICD-10-CM codes: E10.x–E13.x) at least 30 days apart during the baseline period or on the index date, or ≥ 1 dispensing for an antidiabetic medication and ≥ 1 diagnosis of diabetes during the baseline period or on the index date; and (5) ≥ 18 years of age at the index date.
Study design. Data source: Optum’s de-identified Clinformatics® Data Mart Database, consisting of patients with AF, from January 1, 2012, to September 30, 2021. AF atrial fibrillation; GPI generic product identifier; ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM International Classification of Diseases, Tenth Revision, Clinical Modification; NVAF non-valvular atrial fibrillation; HRU healthcare resource use1GPI drug codes were used to identify pharmacy claims for rivaroxaban and warfarin2Treatment discontinuation is defined as a gap in days of supply (i.e., > 30 days) between the end of a dispensing (based on days of supply) and the next fill or between the end of the last dispensing and the end of data3AF was identified with the following ICD-9-CM codes: 427.31, or ICD-10-CM: I48.0, I48.1x, I48.2x, I48.914Diabetes was identified as ≥ 2 diagnosis of diabetes (ICD-9-CM codes: 250.x, or ICD-10-CM codes: E10.x–E13.x) at least 30 days apart during the baseline period or on the index date, or ≥ 1 dispensing for an antidiabetic medication and ≥ 1 diagnosis of diabetes during the baseline period or on the index date
Patients were excluded if they met at least one of the following criteria: (1) dispensings for ≥ 2 oral anticoagulants on the index date, (2) ≥ 1 dispensing for an oral anticoagulant during the baseline period, or (3) ≥ 1 diagnosis of another indication for oral anticoagulation (i.e., venous thromboembolism, knee or hip replacement surgery; in any position) during the baseline period or on the index date. Additionally, to identify NVAF, patients were excluded if they had ≥ 1 diagnosis of mitral stenosis (in any position) during the baseline period or on the index date, or if they had ≥ 1 mechanical heart-valve procedure during the baseline period or on the index date.
Patients meeting the study selection criteria were assigned to mutually exclusive rivaroxaban and warfarin cohorts based on the oral anticoagulant received on the index date. Patients’ demographics and clinical characteristics were evaluated during the 12-month baseline period. An on-treatment approach was used to evaluate HRU and healthcare cost outcomes from the index date up to 12 months (as the main analysis) and up to 24 months (as a sensitivity analysis) post-index. In this approach, the observation (follow-up) period spanned from the index date until the earliest date of treatment discontinuation [defined as the earliest of a gap in days of supply (i.e., > 30 days) between the end of a dispensing (based on days of supply) and the next fill, or between the end of the last dispensing and the end of eligibility], a switch to or addition of another oral anticoagulant, 12 months post-index (or 24 months for sensitivity), death, health plan disenrollment, or end of data availability.
Study Outcomes
The study outcomes were measured up to 12 months post-index as a main analysis and up to 24 months post-index as a sensitivity analysis. All-cause HRU components were evaluated and comprised IP stays (including hospitalizations and skill nursing facility stays), length of stay (measured in days among patients with IP stays), emergency room (ER) visits, and OP visits. Additionally, this study evaluated 30 day readmissions, defined as IP stays during the observation period that were followed by an unplanned acute readmission for any diagnosis within 30 days following the discharge date from the initial IP stay. For all-cause healthcare costs, total costs were calculated as the sum of medical and pharmacy costs, where medical costs included costs associated with IP stays, ER visits, and OP visits.
Statistical Analyses
The inverse probability of treatment weighting (IPTW) approach was used and standardized to the rivaroxaban cohort to estimate an average treatment effect on the treated (ATT) [41]. Weights were calculated based on a propensity score (PS), defined as the conditional probability of being treated with rivaroxaban based on observable baseline characteristics. The PS was then used to create a pseudo-population such that the distribution of covariates in the control group (i.e., warfarin cohort) mimicked the distribution of covariates in the treatment group (i.e., rivaroxaban cohort) [42]. The probability weight was 1 for patients in the rivaroxaban cohort, and calculated as PS/1–PS for those in the warfarin cohort, and then normalized (i.e., dividing each weight by the mean of the weights per cohort) to preserve cohort size.
Baseline characteristics used in the PS calculation included age, sex, year of index date, region, insurance plan type, Quan-Charlson comorbidity index (CCI) score, CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack [TIA], vascular disease, age 65–74 years, sex category) score, HAS-BLED (hypertension, abnormal renal and liver function, stroke, bleeding) score, diabetes complications severity index (DCSI) score, DCSI-related complications (i.e., cardiovascular complications, nephropathy, neuropathy, peripheral vascular disease, cerebrovascular complications, and retinopathy), stroke/systemic embolism (SE), major bleeding, baseline medication use (i.e., non-oral anticoagulants, antihypertensives, antihyperlipidemics, other cardiovascular agents, antiplatelets, and antidiabetics), cardiovascular procedures (i.e., percutaneous coronary intervention, catheter ablation, and coronary bypass graft), other comorbidities of interest with prevalence ≥ 5%, history of cancer diagnosis and treatment, HRU (IP, ER, and OP), and costs (IP, ER, OP, and pharmacy). To prevent outliers from skewing the results of our analyses, observations assigned extremely high weights were truncated at the 99th percentile of the distribution, whereby all weights higher than the 99th percentile value were replaced by that threshold value.
Patients’ baseline characteristics (unweighted and weighted) were reported by treatment cohort using descriptive statistics. Differences in baseline characteristics between cohorts were assessed using standardized differences. A standardized difference < 10% was considered a negligible difference between cohorts [43].
HRU rates and healthcare costs were reported per patient-year (PPY), calculated as the number of events and the costs divided by the patient-years of observation, respectively. This approach is commonly used in non-experimental study settings to account for different lengths of observation periods between patients. HRU rates were compared between cohorts using weighted rate ratios (RR) obtained from Poisson regression models and weighted odds ratios (OR) from logistic regression models. Healthcare costs from a payers’ perspective were reported as the weighted mean (standard deviation [SD]), and weighted cost differences between cohorts were calculated. All costs were inflated to 2021 US dollars based on the medical care component of the Consumer Price Index [44]. Because HRU and cost data have positive values that follow a non-normal distribution and also often have zero values, non-parametric bootstrap procedures were used to estimate 95% confidence intervals (CI) and p values [45]. All analyses were conducted using SAS Enterprise Guide v.7.15.
Results
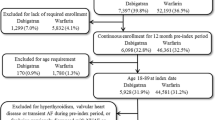
A total of 1,092,499 patients initiated on an oral anticoagulant (rivaroxaban or warfarin) between January 01, 2013, and September 30, 2021, were identified. After applying all inclusion and exclusion criteria, a total of 37,155 patients with NVAF and diabetes were included (17,881 patients initiating rivaroxaban and 19,274 patients initiating warfarin treatment; Fig. 2).
Patient disposition. Data Source: Optum’s de-identified Clinformatics® Data Mart Database, consisting of patients with NVAF and diabetes, from January 1, 2012, to September 30, 2021. AF atrial fibrillation; GPI generic product identifier; ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM International Classification of Diseases, Tenth Revision, Clinical Modification; NVAF non-valvular atrial fibrillation; VTE venous thromboembolism1Baseline period was defined as the 12 months prior to the index date (excluding the index date)2AF was identified with the following codes: ICD-9-CM: 427.31; ICD-10-CM: I48.0, I48.1x, I48.2x, I48.913Diabetes was identified with the following codes ICD-9-CM: 250.x; ICD-10-CM: E10.x, E11.x, E12.x, E13.x
Baseline Demographics and Clinical Characteristics
Baseline characteristics of the unweighted and weighted rivaroxaban and warfarin cohorts are shown in Tables 1 and 2. After weighting, the rivaroxaban and warfarin cohorts were well-balanced with respect to demographics, clinical characteristics, and comorbidities. After weighting, the mean age of the rivaroxaban and warfarin cohorts was 73 years and roughly 40% of patients in each cohort were female (Table 1). Most patients in the rivaroxaban and warfarin cohorts had Medicare Advantage plans and used cardiovascular-related and diabetes medications. The mean (SD) total all-cause healthcare costs during the baseline period were $43,042 ($66,848) for the rivaroxaban cohort and $43,182 ($65,605) for the warfarin cohort. Patients in the rivaroxaban and warfarin cohorts were similar in terms of their mean CCI score, CHA2DS2-VASc score, and HAS-BLED score (Table 2).
Comparisons of HRU
In the main analysis with up to 12 months of follow-up, the mean rate of all-cause HRU was significantly lower in the rivaroxaban versus warfarin cohort across all observed components (Fig. 3). Over a mean (median) follow-up time of 215 (217) days and 214 (218) days for the weighted rivaroxaban and warfarin cohorts, respectively, patients in the rivaroxaban cohort had significantly lower rates of IP stays (RR: 0.84, 95% CI 0.81, 0.88; p < 0.001), ER visits (RR: 0.87, 95% CI 0.79, 0.94; p < 0.001), and OP visits (RR: 0.67, 95% CI 0.66, 0.68; p < 0.001) compared to the warfarin cohort. With respect to IP stays, rivaroxaban was associated with a significantly lower proportion of patients with 30-day readmission (4.0% vs. 5.3%; OR: 0.75, 95% CI 0.66, 0.83; p < 0.001) relative to warfarin.
Healthcare Resource Utilization among weighted rivaroxaban and warfarin cohorts, up to 12 months of follow-up1. CI confidence intervals; ER emergency room; OP outpatient; *p < 0.001; 1The observation period spans from the index date up to the treatment discontinuation, a switch to/addition of another oral anticoagulant, 12 months post-index, death, end of eligibility, or end of data availability, whichever occurs first2Rate ratios are calculated from Poisson regression models3CI and p values are generated using a non-parametric bootstrap procedure with 499 replications
A sensitivity analysis with up to 24 months of follow-up yielded a comparable pattern of results to the main analysis (Figure S1 in Supplementary Material).
Comparisons of Healthcare Costs
Results of the all-cause healthcare cost analysis are shown in Fig. 4. Medical cost savings associated with rivaroxaban (mean cost difference PPY: − $9306, 95% CI − $11,769, − $6607; p < 0.001) fully offset its higher pharmacy costs relative to warfarin (mean cost difference PPY: $5518, 95% CI $5193, $5839; p < 0.001), resulting in significantly lower total all-cause healthcare costs in the rivaroxaban versus warfarin cohort (mean cost difference PPY: − $3788, 95% CI − $6258, − $1035; p < 0.001). The lower medical costs among patients initiated on rivaroxaban compared to those initiated on warfarin were driven by reductions in IP stay costs (mean cost difference PPY: − $4554, 95% CI − $6449, − $2645; p < 0.001) and OP visit costs (mean cost difference PPY: − $4215, 95% CI − $5599, − $2742; p < 0.001). Relative to patients in the warfarin cohort, patients in the rivaroxaban cohort also had significantly lower ER visit costs (mean cost difference PPY: − $537, 95% CI − $912, − $156; p = 0.004).
Healthcare Costs among weighted rivaroxaban and warfarin cohorts, up to 12 months of follow-up1. CI confidence intervals; ER emergency room; OP outpatient; PPY per patient-year1The observation period spans from the index date up to the treatment discontinuation, a switch to/addition of another oral anticoagulant, 12 months post-index, death, end of eligibility, or end of data availability, whichever occurs first2CI and p values are generated using a non-parametric bootstrap procedure with 499 replications
Consistent with the main analysis, total all-cause healthcare costs were significantly lower in the rivaroxaban versus warfarin cohort up to 24 months of follow-up (Figure S2 in Supplementary Material).
Discussion
To our knowledge, this is the first retrospective cohort study comparing the HRU and healthcare costs associated with rivaroxaban and warfarin among newly initiated patients with NVAF and diabetes in the US, irrespective of body weight. Results of this study found that rivaroxaban was associated with a lower all-cause HRU and healthcare cost burden relative to warfarin. Compared to patients in the warfarin cohort, those in the rivaroxaban cohort had significantly lower rates of IP stays, OP visits, and lower odds of 30-day hospital readmissions, which translated into significantly lower total healthcare costs for the rivaroxaban versus warfarin cohort. In particular, while pharmacy costs were higher for the rivaroxaban cohort, this expense was fully offset by medical cost savings relative to the warfarin cohort. In a sensitivity analysis, this cost offset due to medical cost savings among the rivaroxaban cohort was sustained for up to 24 months of follow-up. Taken together, these results highlight the long-term incremental economic benefits of rivaroxaban over warfarin among complex patients with NVAF and diabetes.
These findings are critical given a trend toward increased hospitalizations and costs of care among patients with NVAF in the US over the past two decades [46], along with evidence that comorbid diabetes may exacerbate this burden [6, 7, 47]. Based on one prior study of Medicare beneficiaries with NVAF, hospitalization rates have increased by approximately 1% per year between 1999 and 2013 [46]. Although this study found that the median length of hospital stay has remained stable over time, median Medicare IP expenditure per beneficiary has increased from $2932 to $4719 per hospital stay [46]. In another study using data from a nationwide readmission database from 2010 to 2014, an estimated 1 in 7 patients with NVAF were readmitted within 30 days of discharge, with readmissions accounting for a significant 3% increase in the costs of care per patient [47]. In addition, this study found that comorbidities such as diabetes mellitus were found to be highly predictive of 30-day readmissions and were associated with increased hospitalization costs [47]. In light of these trends, rivaroxaban may play an important role in mitigating the cost burden associated with increased hospitalizations, including 30-day readmissions, among patients with NVAF and diabetes from a US healthcare payer perspective.
The present study findings are consistent with the pattern observed in prior retrospective analyses of anticoagulant users with NVAF overall. Among matched patients from the general NVAF population, those initiated on rivaroxaban had a significantly reduced number and length of all-cause hospitalizations and fewer OP visits, resulting in significantly lower associated costs compared to those initiated on warfarin [29, 30]. The present findings also corroborate prior evidence showing that rivaroxaban maintains this economic advantage over warfarin, even among NVAF patients with common and burdensome comorbidities [33,34,35]. To date, no studies have specifically examined HRU and costs associated with anticoagulation therapy in anticoagulant-naïve patients with NVAF and diabetes. However, a recent retrospective study by Weir et al. assessed these outcomes among commercially-insured patients with concurrent NVAF, obesity, and diabetes [36]. In this study, rivaroxaban was associated with significant reductions in all-cause total healthcare costs (cost difference: − $7816 per PPY) compared to warfarin over a mean follow-up time of approximately 30 months [36]. Reductions in hospitalizations and reduced 30-day readmissions in the rivaroxaban cohort contributed to significantly lower medical costs, which fully compensated for higher pharmacy costs relative to the warfarin cohort [36]. The present study observed a similar pattern of results to Weir et al. [36], suggesting that rivaroxaban reduces the HRU and cost burden among NVAF patients with diabetes irrespective of body weight. Finally, our sensitivity analysis showed a sustained reduction in healthcare costs due to medical cost savings with rivaroxaban at up to 24 months of follow-up, which is also consistent with the findings by Weir et al. [36] over a 30-month period, as well as the findings of prior studies showing economic benefits of rivaroxaban over warfarin at up to 36 months of follow-up [34, 35].
These HRU and cost reductions associated with rivaroxaban could potentially reflect differences in its clinical profile relative to warfarin based on evidence from real-world clinical practice [29, 30, 48,49,50]. In prior studies among NVAF patients with diabetes, rivaroxaban has been associated with improved clinical outcomes along with HRU benefits relative to warfarin [26, 27]. Given that all-cause HRU and medical costs in the present study also included those associated with stroke/SE and bleeding events, it is plausible that part of the HRU/cost reductions in the rivaroxaban cohort may have been driven by improved clinical outcomes relative to the warfarin cohort. In one recent claims-based study of NVAF patients with type 2 diabetes, rivaroxaban was associated with a lower risk of stroke/SE, ~ 10% relative reduction in vascular mortality, and fewer bleeding-related hospitalizations versus warfarin [26]. Moreover, DOACs such as rivaroxaban may help to mitigate the impact of acute or chronic kidney disease, which is a common complication of diabetes [51]. In one real-world study of NVAF patients with diabetes, rivaroxaban was associated with a lower risk of hospital admissions/ER visits for acute kidney injury, as well as with a reduced risk of other undesirable renal outcomes when compared to warfarin [27]. Future research is needed to elucidate the factors driving the HRU and cost reductions associated with rivaroxaban, as well as the relationship between clinical and economic outcomes among anticoagulant users with NVAF and diabetes.
Limitations
Limitations of the present study include its observational and retrospective nature, as well as the limitations commonly associated with claims databases, such as the potential for coding inaccuracies that could lead to case misidentification and missing data. Relatedly, some details are unavailable in claims data, such as clinical information (e.g., disease severity) and certain treatment-related information (e.g., over-the-counter medications), while clinical events leading to death before reaching a medical facility may not be observed in the data either. Moreover, the healthcare costs included in the CDM database are standardized costs that reflect estimated allowed payments across all provider services to account for differences in pricing across health plans and provider contracts; however, this standard pricing algorithm could lead to underestimation or overestimation of costs. Although IPTW was used to adjust for observed differences between rivaroxaban and warfarin cohorts, the possibility of unobserved confounding (e.g., patients' diet, exercise) and confounding by indication cannot be excluded. This study included patients diagnosed with either type of diabetes (1 or 2); the relative impact of rivaroxaban versus warfarin on HRU and costs was not separately evaluated among subgroups of patients with type 1 or type 2 diabetes. Finally, the study results may not be generalizable to other patient populations (e.g., those enrolled in Medicaid or those without health insurance).
Conclusions
The present study provides important insights into the economic burden of patients with NVAF and diabetes irrespective of body weight, and clarifies the impact of anticoagulant therapies on this burden. Among NVAF patients with diabetes initiated on anticoagulant therapy, rivaroxaban was associated with significantly lower HRU compared to warfarin, most notably lower rates of hospitalization and odds of 30-day readmission. Rivaroxaban was also associated with significantly lower total all-cause healthcare costs when compared to warfarin, with significant medical cost savings fully offsetting higher pharmacy costs. This suggests that rivaroxaban may play an important role in curbing the substantial HRU and cost burden associated with NVAF and diabetes in the US.
References
Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study JAMA. 1994;271(11):840–4.
Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108(1):56–62.
Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care. 2009;32(10):1851–6.
Xiong Z, Liu T, Tse G, Gong M, Gladding PA, Smaill BH, et al. A machine learning aided systematic review and meta-analysis of the relative risk of atrial fibrillation in patients with diabetes mellitus. Front Physiol. 2018;9:835.
Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30(9):1128–35.
Echouffo-Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, et al. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF registry. J Am Coll Cardiol. 2017;70(11):1325–35.
Kumar N, Echouffo-Tcheugui JB. Diabetes and atrial fibrillation in hospitalized patients in the United States. Clin Cardiol. 2021;44(3):340–8.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations) a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(18):1935–44.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–32.
Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127(4):e15–6.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–88.
Electronic Medicines Compendium. SPC. Warfarin 0.5 mg tablets. Accessed on: Nov 22. Available from: Available from: https://www.medicines.org.uk/emc/medicine/27651.
United States Food and Drug Administration. ELIQUIS® (apixaban)—prescribing information. Accessed on: June 13. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202155s034lbl.pdf.
United States Food and Drug Administration. PRADAXA® (dabigatran etexilate)—Prescribing Information Accessed on: June 13. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022512s041lbl.pdf.
United States Food and Drug Administration. XARELTO® (rivaroxaban)—Prescribing Information. Accessed on: June 8. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022406Orig1s039,202439Orig1s038correctedlbl.pdf.
Coleman CI, Briere JB, Fauchier L, Levy P, Bowrin K, Toumi M, et al. Meta-analysis of real-world evidence comparing non-vitamin K antagonist oral anticoagulants with vitamin K antagonists for the treatment of patients with non-valvular atrial fibrillation. J Mark Access Health Policy. 2019;7(1):1574541.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91.
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51.
Patti G, Di Gioia G, Cavallari I, Nenna A. Safety and efficacy of nonvitamin K antagonist oral anticoagulants versus warfarin in diabetic patients with atrial fibrillation: a study-level meta-analysis of phase III randomized trials. Diabetes Metab Res Rev. 2017;33(3):e2876.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62.
Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, et al. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: the rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF trial). Am Heart J. 2015;170(4):675–828.
Coleman CI, Costa OS, Brescia CW, Vardar B, Abdelgawwad K, Sood N. Thromboembolism, bleeding and vascular death in nonvalvular atrial fibrillation patients with type 2 diabetes receiving rivaroxaban or warfarin. Cardiovasc Diabetol. 2021;20(1):52.
Hernandez AV, Bradley G, Khan M, Fratoni A, Gasparini A, Roman YM, et al. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):301–7.
Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–20.
Laliberte F, Cloutier M, Crivera C, Nelson WW, Olson WH, Schein J, et al. Effects of rivaroxaban versus warfarin on hospitalization days and other health care resource utilization in patients with nonvalvular atrial fibrillation: an observational study from a cohort of matched users. Clin Ther. 2015;37(3):554–62.
Laliberte F, Pilon D, Raut MK, Nelson WW, Olson WH, Germain G, et al. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin. 2014;30(8):1521–8.
Milentijevic D, Lin JH, Chen YW, Kogan E, Shrivastava S, Sjoeland E, et al. Healthcare costs before and after stroke in patients with non-valvular atrial fibrillation who initiated treatment with rivaroxaban or warfarin. J Med Econ. 2021;24(1):212–7.
Mittal VS, Wu B, Song J, Milentijevic D, Ashton V, Mahajan D. Healthcare resource utilization and costs among nonvalvular atrial fibrillation patients initiating rivaroxaban or warfarin in skilled nursing facilities: a retrospective cohort study. Curr Med Res Opin. 2020;36(4):529–36.
Peterson ED, Ashton V, Chen YW, Wu B, Spyropoulos AC. Comparative effectiveness, safety, and costs of rivaroxaban and warfarin among morbidly obese patients with atrial fibrillation. Am Heart J. 2019;212:113–9.
Berger JS, Laliberte F, Kharat A, Lejeune D, Moore KT, Jung Y, et al. Healthcare resource utilization and costs of rivaroxaban versus warfarin among non-valvular atrial fibrillation (NVAF) patients with obesity in a US population. J Med Econ. 2021;24(1):550–62.
Laliberte F, Ashton V, Kharat A, Lejeune D, Moore KT, Jung Y, et al. Economic burden of rivaroxaban and warfarin among nonvalvular atrial fibrillation patients with obesity and polypharmacy. J Comp Eff Res. 2021;10(16):1235–50.
Weir MR, Chen YW, He J, Bookhart B, Campbell A, Ashton V. Healthcare resource utilization and costs of rivaroxaban versus warfarin among nonvalvular atrial fibrillation patients with obesity and diabetes. Diabetes Ther. 2021;12(12):3167–86.
Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020: Estimates of Diabetes and Its Burden in the United States. Accessed on: Nov 29. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
Gujral UP, Narayan KMV. Diabetes in normal-weight individuals: high susceptibility in nonwhite populations. Diabetes Care. 2019;42(12):2164–6.
Gregg EW, Cadwell BL, Cheng YJ, Cowie CC, Williams DE, Geiss L, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27(12):2806–12.
Optum Inc. Clinformatics® Data Mart User Manual. 2020(8.1):1–41.
Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–55.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17(12):1202–17.
United States Bureau of Labor Statistics. Consumer Price Index. Accessed on: May 20. Available from: https://www.bls.gov/cpi/.
Afifi AA, Kotlerman JB, Ettner SL, Cowan M. Methods for improving regression analysis for skewed continuous or counted responses. Annu Rev Public Health. 2007;28:95–111.
Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for medicare beneficiaries, 1999–2013. Circulation. 2017;135(13):1227–39.
Tripathi B, Atti V, Kumar V, Naraparaju V, Sharma P, Arora S, et al. Outcomes and resource utilization associated with readmissions after atrial fibrillation hospitalizations. J Am Heart Assoc. 2019;8(19): e013026.
Laliberte F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30(7):1317–25.
Bai Y, Deng H, Shantsila A, Lip GY. Rivaroxaban versus dabigatran or warfarin in real-world studies of stroke prevention in atrial fibrillation: systematic review and meta-analysis. Stroke. 2017;48(4):970–6.
Milentijevic D, Lin JH, Connolly N, Chen YW, Kogan E, Shrivastava S, et al. Risk of stroke outcomes in atrial fibrillation patients treated with rivaroxaban and warfarin. J Stroke Cerebrovasc Dis. 2021;30(5): 105715.
Kreutz R, Camm AJ, Rossing P. Concomitant diabetes with atrial fibrillation and anticoagulation management considerations. Eur Heart J Suppl. 2020;22(Suppl O):O78–86.
Acknowledgements
Funding
This study was funded by Janssen Scientific Affairs, LLC. The study sponsor was involved in several aspects of the research, including the study design, interpretation of data, and approval of the final manuscript. The study sponsor also funded the journal's Rapid Service and Open Access Fees.
Medical Writing Assistance
Medical writing assistance was provided by professional medical writer, Mona L. Chanda, PhD, an employee of Groupe d’analyse, Ltée, a consulting company that has provided paid consulting services to Janssen, which funded the development and conduct of this study and manuscript.
Author Contributions
All authors were involved in the conception and design, or analysis and interpretation of the data; the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published.
Disclosures
Jeffrey S. Berger and Matthew R. Weir received consultancy fees from Janssen Scientific Affairs, LLC. Veronica Ashton and Brahim Bookhart are employees of Janssen Scientific Affairs, LLC who may own stock or stock options. François Laliberté, Guillaume Germain, Dominique Lejeune, Julien Boudreau, and Patrick Lefebvre are employees of Groupe d’analyse, Ltée, a consulting firm that received consulting fees from Janssen Scientific Affairs, LLC, for the conduct of this study.
Compliance with Ethics Guidelines
Data were de-identified and comply with the patient requirements of the HIPAA of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) (https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/#46.101). Furthermore, no consent to participate or for publication was required. Finally, this study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The data that support the findings of this study are available from Optum’s CDM. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from www.optum.com with the permission of Optum.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Berger, J.S., Ashton, V., Laliberté, F. et al. Healthcare Resource Utilization and Costs of Rivaroxaban Versus Warfarin Among Non-valvular Atrial Fibrillation (NVAF) Patients with Diabetes in a US Population. Adv Ther 40, 1224–1241 (2023). https://doi.org/10.1007/s12325-022-02422-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02422-9