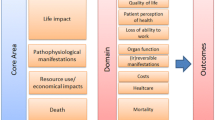
Abstract
Introduction
Hemorrhoidal disease (HD) is characterized by prolapse of the inflamed and bleeding vascular tissues of the anal canal. Although HD is associated with a high recurrence rate, there is a lack of understanding around interventions that can reduce recurrence and improve outcomes for patients. As such, a systematic literature review (SLR) was conducted to summarize evidence on epidemiology, recurrence, and efficacy of interventions in HD.
Methods
Real-world evidence (RWE) studies evaluating the incidence, prevalence, or recurrence of HD, as well as SLRs including a meta-analytic component reporting on the efficacy of systemic or topical pharmacological treatments for adults with HD, were included. Systematic searches were conducted in MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature, and the Cochrane Database of Systematic Reviews.
Results
The SLR identified 44 eligible publications. Consistent data were limited on the epidemiology of HD or HD recurrence. Specifically, incidence and prevalence reported across geographies were impacted by differences in data collection. Reported risk factors for HD were sedentary behavior, constipation, male gender, and age. Twenty-three RWE studies and one meta-analysis reported HD recurrence rates ranging from 0 to 56.5% following surgery or phlebotonics, with most (n = 19) reporting rates of 20% or less. In addition to time since treatment, risk factors for recurring disease were similar to those for HD in general. With respect to treatment, micronized purified flavonoid fractions significantly improved the main symptoms of HD compared to other pharmacological treatments.
Conclusion
The SLRs did not identify any RWE studies reporting recurrence in patients receiving systemic or topical treatments, highlighting the need for future research in this area. Further, more studies are needed to understand the optimum duration of medical treatment to prevent recurrence.
Graphical abstract

Plain Language Summary
Patients with hemorrhoidal disease (HD) can experience recurring disease following a period of improvement or remission. It is not well established how often this might happen, who is at greatest risk, or which treatments can reduce this risk. In this study, a systematic literature review (SLR) was conducted to summarize evidence on the occurrence and recurrence of HD, as well as treatment effectiveness. Several literature databases were searched for articles that described real-world evidence (RWE) studies reporting the epidemiology or recurrence of HD as well as published SLRs that combined the results of multiple studies (meta-analyses) on treatment for adults with HD. Forty of 2037 articles identified by the search were considered relevant, and four others identified by clinicians were also included (total = 44; 39 RWE, 5 meta-analyses). Review of the RWE articles revealed that HD epidemiology was determined differently between studies. Only 23 reported recurrence rates (up to 56.5%) after surgery or treatment with phlebotonic drugs (drugs that improve blood flow in veins). Most (19/23) reported recurrence rates of 20% or less. Risk factors for recurrence were similar to usual HD risk factors (e.g., constipation, male gender, age) in addition to time since treatment. Phlebotonic agents, including those made from plant extracts (micronized purified flavonoid fractions, MPFFs) improved hemorrhoidal symptoms compared with placebo or no treatment. In one meta-analysis, MPFF was the only phlebotonic to significantly reduce recurrence risk versus no treatment or placebo. Overall, more research is needed to compare treatments and determine optimal treatment duration to prevent recurrence.
Author-narrated video abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A systematic literature review was conducted to summarize evidence on the epidemiology and recurrence of hemorrhoidal disease. |
Consistent data were limited on the topics of interest. |
Recurrence rates varied across the included real-world evidence studies, ranging from 0% to 56.5% following surgery or other procedural treatment. |
Additional research is needed to evaluate comparative efficacy for reduction in recurrence between two active treatments. |
Digital Features
This article is published with digital features, including video abstract and infographic, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21277593.
Introduction
Hemorrhoidal disease (HD) is characterized by prolapse of the inflamed and bleeding vascular tissues of the anal canal and is associated with a high recurrence rate as well as a considerable burden to individuals.
International consensus on individualized, effective disease management has not been reached. Treatment options for HD range from conservative methods (e.g., dietary and lifestyle modifications) and medical management (e.g., venoactive drugs [also known as phlebotonics]) to non-invasive procedures such as sclerotherapy, rubber band ligation, infrared coagulation, radiofrequency ablation, or invasive surgery procedures. Although recurrence commonly occurs in people with HD, there is a lack of understanding around the rates of recurrence, and interventions that can reduce this and improve outcomes for patients. A further understanding of recurrence rates in HD could support clinical decision-making around the best treatments.
The objective of this systematic literature review (SLR) was to summarize studies evaluating the epidemiology of HD, including incidence, prevalence, recurrence rates, and risk factors for HD and recurrence of HD, and to assess the efficacy/effectiveness of various systemic or topical pharmacological treatments for adults with HD on the basis of previously published SLRs that included a meta-analytic component.
Methods
This SLR was conducted following standards from the Cochrane Collaboration [1] and reporting standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [2]. The topics of interest reported herein were part of a broader SLR focused on the overall burden of HD, which included literature on diagnosis, epidemiology, humanistic burden, economic burden, and disease management, as well as clinical efficacy and safety of treatment for HD.
This article is based on previously conducted studies; none of the authors performed any new studies involving human participants or animals. No analyses were conducted.
Eligibility Criteria
Studies meeting the following criteria were included: (1) Real-world studies reporting epidemiology (including incidence, prevalence, recurrence rates, and risk factors) of adults with HD as reported for key regions of interest in Europe, North America, Asia–Pacific, as well as Brazil, Egypt, and Türkiye (see Appendix in the supplementary material); (2) SLRs that included a meta-analytic component reporting on the efficacy/effectiveness of systemic or topical pharmacological treatments (alone or in combination with other therapies) for adults with HD.
SLRs focusing on invasive or surgical procedures were excluded. There were no restrictions with respect to disease stage. Epidemiology studies were limited to those published in 2009 or later; there was no time limit for published SLRs.
Further details regarding the population, intervention, comparator, outcomes, and study designs of interest are presented in the Appendix.
Study Identification
Systematic database searches were conducted via Ovid SP on 13 August 2019, including MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Database of Systematic Reviews. The search strategies (Tables S1, S2, and S3 in the supplementary material) were designed using a combination of Medical Subject Heading, Emtree, and free-text terms for HD paired with validated filters to identify studies reporting on all topics of interest in the broader review (epidemiology, diagnostics, quality of life, and clinical and economic burden). The search strategies are provided in the online Appendix.
Study Selection
Each title and abstract were reviewed by one investigator to determine the article’s suitability for inclusion in the review, according to the defined inclusion and exclusion criteria (Table S4 in the supplementary material). A second, independent investigator reviewed 10% of the abstracts as a quality check. Discrepancies between the first and second reviewers were resolved by a third, senior investigator. For abstracts that were deemed relevant, the corresponding full-text articles were retrieved for further evaluation. Each full-text article was reviewed by one investigator. All rejected articles were assigned a reason for exclusion and were validated by a second, independent investigator. Discrepancies were resolved by a third, senior investigator.
Data Extraction and Reporting
Data extraction was conducted by one investigator with all extractions validated by a second investigator. Study, population, and treatment characteristics, as well as outcomes of interest, from selected full-text articles were extracted into a pre-defined data extraction table. Results were summarized qualitatively; no analyses were performed.
The quality of the included real-world evidence (RWE) studies was assessed using the modified Downs and Black checklist, and each was scored out of 28 [3].
Assessment of Risk of Bias
The methodological quality of the included SLRs was assessed using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR) 2 tool [4]. The AMSTAR 2 checklist includes 16 items to assess specific elements of the conduct of SLRs, seven of which are considered critical. The quality of included RWE studies reporting on recurrence rates was assessed using the modified Downs and Black checklist [3].
Results
PRISMA
Systematic searches for the review identified 2037 records from electronic literature databases. After the removal of 458 duplicates, 1579 remained for title and abstract screening. Of these, 443 publications were deemed eligible for screening at the full-text level, of which 40 met the inclusion criteria of the review. An additional four records were identified by clinical experts, resulting in 44 total records ultimately included in the review (Fig. 1).
Characteristics of Included Studies
Thirty-nine RWE studies were identified which reported on the epidemiology of HD, of which two reported incidence, five reported prevalence, and 14 reported risk factors of HD. A snapshot of included studies is reported in Table 1. Five SLRs provided data on the clinical efficacy or safety of treatment for HD (Table 2). Twenty-three of the included RWE studies also reported on recurrence of disease, all of which reported recurrence following procedural treatments. A single SLR reported recurrence rates following systemic treatment.
Twenty-one RWE studies took place in Europe, five in China, five in the USA, two in Australia, two in Türkiye, and one each in Taiwan and Brazil. One RWE study was international, and one was conducted in the USA and Puerto Rico (Fig. 2). The included SLRs were published between 2006 and 2018. Databases searched within the SLRs included PubMed/MEDLINE (n = 4), Embase (n = 4), Cochrane Library (n = 4), and CINAHL (n = 3). All SLRs included only clinical trial evidence.
The quality of most RWE studies was in the 10- to-15-point range out of 28. Five SLRs were assessed per AMSTAR 2 criteria—one was judged to be viewed with low confidence, two with critically low confidence, and two with moderate confidence. Three of the five SLRs failed to assess the impact of publication bias on their results (AMSTAR 2 criterion 15) and did not have a protocol available (AMSTAR 2 criterion 3).
Epidemiology
Thirty-nine RWE studies reporting on the incidence, prevalence, risk factors, and recurrence of HD were included in the SLR (Table S5 in the supplementary material). The characteristics of 10 of the most representative epidemiology studies, by geography, are reported in Table 1.
Prevalence and Incidence of HD
Prevalence based on 2011 data from a convenience sample of 39 general practices across metropolitan France and some overseas territories was 5.84% [5]. The annual prevalence rate was 1.2% among 18.9 million adults with at least one HD-related claim enrolled in the US Truven claims database in 2014 [14].
Prevalence rates were greater in at-risk individuals. Increased sitting (≥ 9 h/day) was associated with a nonsignificant increase in HD prevalence in a multivariate analysis (odds ratio [OR] 1.18; 95% CI 0.92, 1.50; p = 0.08) [12]. In Brazil, HD was approximately 4.5 times more prevalent in women with vaginal deliveries compared to those who underwent caesarean deliveries [10]. Cesarean deliveries were found to be protective against hemorrhoids with a prevalence ratio of 0.16 (95% CI 0.04, 0.57) when adjusted for age, type of provider, number of prenatal consultations, parity, and hypertension and diabetes during pregnancy [10].
Abramowitz et al. presented incident cases diagnosed and reported by 39 primary care physicians in France during 2.5 days of consultation in 2010. Among 1079 patients, 74 presented with a new diagnosis of HD (6.9% or 69 per 1000 persons) [5]. Lin et al. reported on the new HD diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification: 455) among patients with and without chronic obstructive pulmonary disease (COPD) as reported in a Taiwanese national database between 2000 and 2003 and followed up until 2011 [9]. Overall incidence among patients with COPD was approximately 1.6 times greater than in patients without COPD (average annual incidence 11.5 vs. 7.1 per 1000 person-years) [9]. These findings were consistent between men and women, and across different age groups [9]. Neither study was designed specifically to measure HD incidence in the general population, and thus estimates are likely to be imperfect. The dissimilar estimates may be due to methodology of data collection, or may reflect true differences; additional information is needed to understand the real incidence (69/1000 vs. 7.1/1000) of HD.
Risk Factors for HD
A cross-sectional study was conducted by Peery et al. in patients recruited from 11 centers in the USA and Puerto Rico from 2004 to 2008 with satisfactory preparation for colonoscopy; results found that constipation, straining during bowel movements, incomplete bowel emptying, and hard stools were associated with increased risk of hemorrhoids [11]. Additionally, while not statistically significant, high total fiber intake was associated with a reduced risk of hemorrhoids (OR 0.80; 95% CI 0.64, 1.01) [11]. While neither physical activity nor weight was associated with decreased risk, the highest quartile of sedentary behavior (mean 656 min/day) was associated with a lower age- and sex-adjusted risk than the lowest quartile (mean 176 min/day) [11]. An international multivariate analysis by Godeberge et al. examining risk factors in 5617 adults seeking consultation for hemorrhoidal complaints demonstrated that hemorrhoids were significantly associated with age, chronic venous disease (CVD) Classification System for Chronic Venous Disorders (CEAP) class, constipation, and male gender (p < 0.0001 for all) [7].
Clinical Efficacy and Treatments
Five SLRs and meta-analyses of clinical trials reported on the clinical efficacy and safety of treatments for HD including: one SLR for micronized purified flavonoid fractions (MPFF); one SLR for metronidazole; two SLRs for phlebotonics in general in patients with HD; and one SLR for conservative treatments including dietary modifications, bowel transit modifications, local treatments, and drugs in patients with symptomatic hemorrhoids during pregnancy and the puerperium [15,16,17,18,19].
Controls typically included placebo, no treatment, or usual care. One SLR included phlebotonics as a comparator [16] and one SLR did not specify the control group [17]. SLRs differed in terms of the number of trials and patients included, ranging from two to 24 randomized controlled trials (RCT; or 150–2344 patients). Overall, the available evidence across the SLRs was limited and heterogenous, with no single meta-analysis reporting all outcomes of interest.
The SLR by Wanis et al. examined the effect of metronidazole for the treatment of HD pain and found inconsistent efficacy [19]. While metronidazole resulted in statistically significantly lower pain scores than placebo or usual care on the first and fourth days following surgery (standard mean difference [SMD] − 0.87; 95% CI − 1.73, − 0.015; p = 0.046 and − 1.43; 95% CI − 2.83, − 0.037; p = 0.044, respectively) as well as a shorter time to return to normal activities (SMD − 0.76; 95% CI − 1.43, − 0.088; p = 0.027), these results became not significant when the largest trial with the highest risk of bias was removed from the pooled analysis [19].
The SLR by Quijano and Abalos assessed the effectiveness of conservative treatments including dietary modifications, bowel transit modifications, local treatments, and drugs in patients with symptomatic hemorrhoids during pregnancy and the puerperium. Only two studies with rutosides were eligible for the analysis. Rutosides were more likely to produce a treatment response, defined as improvement in symptoms, at 4 weeks post pregnancy when compared with placebo or no treatment (risk ratio [RR] 0.07; 95% CI 0.03, 0.20; p < 0.0001) [18]. However, as there was no difference in maternal outcomes, and the safety data were insufficient to rule out clinically important harms, the authors did not recommend the use of rutosides until further evidence was available [18].
The meta-analysis by Alonso-Coello et al. compared phlebotonics including MPFFs, troxerutin, diosmin, hydroxyethylrutosides with no treatment, or placebo in the treatment of symptomatic HD. Patients treated with phlebotonics were more likely to experience a reduction in bleeding (RR 0.33; 95% CI 0.19, 0.57; p < 0.001) [15] and a significant reduction in pain (RR 0.35; 95% CI 0.18, 0.69; p < 0.001) and itching (RR 0.65; 95% CI 0.44, 0.97; p = 0.01). There was no difference in effect for safety outcomes [15].
Another SLR on phlebotonics by Perera et al., which included mainly studies with MPFFs (but also studies with rutosides, diosmin, troxerutin, pycnogenol, and calcium dobesilate), found a potential benefit in the treatment of acute HD crises as well as a benefit in alleviating post-hemorrhoidectomy symptoms [17]. In this analysis of 24 trials, phlebotonics, particularly MPFFs, statistically significantly improved bleeding (OR 0.12; 95% CI 0.04, 0.37; p = 0.0002), bleeding post-hemorrhoidectomy (OR 0.18; 95% CI 0.06, 0.58; p = 0.004), discharge and leakage (OR 0.12; 95% CI 0.04, 0.42; p = 0.0008), pruritus (OR 0.23; 95% CI 0.07, 0.79; p = 0.02), and overall symptoms (OR 15.99; 95% CI 5.97, 42.84; p < 0.00001) [17]. The use of phlebotonics was associated with a beneficial but not statistically significant effect for pain (OR 0.11; 95% CI 0.01, 1.11; p = 0.06) and for pain scores post hemorrhoidectomy (SMD − 1.04; 95% CI − 3.21, 1.12; p = 0.35). They were also associated with less analgesic consumption (OR 0.54; 95% CI 0.30, 0.99; p = 0.05) as a reflection of their benefit in alleviating pain caused postoperatively.
Finally, the SLR by Aziz et al. specifically examined MPFFs and found a statistically significant improvement in bleeding compared with placebo (RR 1.46; 95% CI 1.10, 1.93; p = 0.008), without a difference between MPFFs and placebo for pain improvement [16]. Itching was improved significantly with MPFFs in two of three studies. As a result of heterogeneity in study designs, endpoints were limited for pooled meta-analyses. Aziz et al. did not perform a pooled analysis of efficacy outcomes comparing MPFFs to other phlebotonics [16].
Recurrence Rates
Twenty-three RWE studies and one meta-analysis [15] reported HD recurrence rates following surgery or phlebotonics. Recurrence rates in the included studies typically ranged from 0 [20, 21] to 56.5% [7] with most (n = 19) reporting rates of 20% or less. Time since procedure was the main driver of this range. Of the studies reporting recurrence, only two prospectively evaluated recurrence rates over time. Ratto et al. found that recurrence of HD requiring surgery after transanal hemorrhoidal dearterialization occurred in 4.1% of patients over a median follow-up of 11.5 months [13]. Conaghan and Farouk found that, among patients with symptomatic grade 2 or 3 HD who failed rubber band ligation and were treated with Doppler-guided hemorrhoid artery ligation, 20% had recurrent symptoms at a median follow-up of 18 months [6].
In addition to time since treatment, risk factors for recurring disease were similar to risk factors for HD in general. In their study of 5617 patients presenting with HD complaints in clinical practice across multiple countries, Godeberge et al. found 56.5% of adults attending a consultation for HD complaints had a previous history of HD [7]. The factors associated with HD recurrence after previous consultation for HD in multivariate analysis included constipation (OR 2.20; 95% CI 1.96, 2.46), age group (OR 2.11; 95% CI 1.68, 2.65 for the comparison of 18–34 years vs. > 65 years), CVD CEAP class (OR 3.75; 95% CI 1.30, 10.90 for the comparison of CEAP C0a vs. C6), body mass index (BMI) category (OR 2.34; 95% CI 1.51, 3.64 for the comparison of 12–18 years vs. ≥ 31 kg/m2), and male gender (OR 1.25; 95% CI 1.11, 1.41) [7]. Among women, having given birth and number of births were the most important risk factors (p < 0.0001), followed by constipation (p < 0.001), age group (p < 0.0001), presence of CVD (p = 0.0089), and BMI category (p = 0.0123) [7]. Among men, constipation was the most important risk factor (p < 0.0001) followed by age group (p < 0.0001), BMI category (p = 0.0011), and presence of CVD (p < 0.0001) [7]. Gender had no influence on likelihood of hemorrhoid recurrence in univariate analysis (p = 0.1539) [7].
In the meta-analysis conducted by Alonso-Coello et al., phlebotonics such as MPFFs, troxerutin, diosmin, hydroxyethylrutosides were compared with no therapy or placebo in the treatment of symptomatic HD. The risk of recurrence was statistically significantly reduced across four studies only for those treated with MPFFs. These recurrences were evaluated for short-to-medium follow-up (2–6 months). The pooled estimate was 47% RR reduction in favor of MPFFs (RR 0.53; 95% CI 0.41, 0.69) [15].
Discussion
This SLR provided evidence regarding the epidemiology of HD and the efficacy of various pharmacological treatments for the condition. Consistent data were limited on the epidemiology of HD or HD recurrence. Specifically, incidence and prevalence reported across geographies were impacted by differences in how data were collected and recorded and sampling methodology. There was also a lack of epidemiology data for HD, and the studies included were heterogeneous in terms of design and target populations. These differences across epidemiological studies hindered the ability to accurately assess the current burden of disease, which is generally acknowledged to be substantial.
A number of studies reported on independent and statistically significant risk factors for HD in general, many of which examined well-established risk factors (e.g., comorbidities and lifestyle aspects). Similar risk factors were identified for HD recurrence, such as constipation and age.
Five included SLRs reported limited information on the efficacy and safety of treatments as a result of heterogeneity of study designs. Generally speaking, metronidazole and rutosides failed to demonstrate superior efficacy when compared with placebo or no treatment, while among phlebotonics, MPFFs significantly improved the main symptoms of HD such as bleeding, discharge/leakage, or pruritus.
These outcomes were supported by a recent meta-analysis which was published after the search period. It assessed 11 RCTs in which MPFF treatment was compared to placebo or no treatment for acute HD or for relief of symptoms after patients had undergone medical management or a surgical procedure to remove hemorrhoids. On the basis of findings from qualitative analysis, MPFFs were reported in most studies to be beneficial in treating bleeding, pain, pruritus, anal discharge/leakage, and tenesmus, and in overall improvement. Quantitative meta-analysis indicated that MPFF treatment provided significant benefits for bleeding (OR 0.082; 95% CI 0.027, 0.250; p < 0.001), discharge/leakage (OR 0.12; 95% CI 0.04, 0.42; p < 0.001), and overall improvement according to patients (OR 5.25; 95% CI 2.58, 10.68; p < 0.001) and investigators (OR 5.51; 95% CI 2.76, 11.00; p < 0.001). MPFF also tended to decrease pain (OR 0.11; 95% CI 0.01, 1.11; p = 0.06). These results suggested MPFF treatment can improve the most important symptoms of HD [22].
While safety events were not a main outcome of interest, three SLRs that focused on phlebotonics (n = 2) or conservative treatment in general (n = 1) found no significant difference between active treatment and placebo with respect to adverse events. Overall, systemic treatment was generally well tolerated with no safety concerns.
Recurrence rates across the included RWE studies ranged from 0 to 56.5% following surgery or other procedural treatment. Factors such as history of HD, time from procedure, and type of procedure played a significant role in HD recurrence. However, differences among populations observed across the RWE studies precluded the ability to draw any strong, meaningful conclusions from the literature. A better understanding of the risk factors and the pathophysiology that trigger the recurrence of HD will help to alleviate further burden on patients.
An included meta-analysis of RCT evidence which involved MPFFs, troxerutin, diosmin, hydroxyethylrutosides found that the risk of recurrence was statistically significantly reduced for those treated with MPFFs compared to placebo or no treatment with 47% risk reduction in favor of MPFFs. This was based on data from four studies out of 14 included RCTs. MPFFs were the only phlebotonic with proven efficacy on the reduction of recurrence of hemorrhoidal symptoms. This finding was further supported by a recently published consensus document which states that the use of MPFFs can help prevent HD recurrence, especially recurrent bleeding [23].
This SLR identified a few gaps in the literature that require additional research. Though 39 RWE studies were included, the SLRs did not identify any reporting recurrence in patients receiving systemic or topical treatments, highlighting the need for more research. Among phlebotonics, MPFF was the most investigated product with the highest quality of evidence. Additional research is also needed to evaluate comparative efficacy for reduction in recurrence between two active treatments. Further, more studies are needed to understand the optimum duration of medical treatment to prevent recurrence.
This research has several limitations; first, there was a general lack of high-quality evidence for RWE studies and limited data available from published SLR/meta-analyses of HD. In particular, only one SLR examined the rate of recurrence of HD after treatment. Second, considerable differences were found across the included studies in how outcome data were defined, collected, measured, and reported, making a direct comparison of treatments or geographic areas difficult. For this reason, consistent measurements, or a set of core outcomes, for efficacy or effectiveness should be used in future studies. Third, the evidence summarized in this report was selected on the basis of a number of additional selection criteria (e.g., sample size, geographic area of interest) to support a meaningful synthesis of results. Finally, the evidence was limited to studies from 2009 onward, with the exception of clinical trials, limiting the ability to identify long-term trends in the data.
This SLR brings added value with several strengths. First, the SLR was conducted in alignment with guidance by the Cochrane Collaboration along with reporting guidelines established by PRISMA. The search strategy used for this SLR was paired with gray literature searches to ensure all relevant evidence was identified. The SLR approach allows for synthesis of evidence across multiple geographies and time periods, creating a broad overview of recurrence rates in HD.
Conclusions
This SLR identified risk factors for HD and HD recurrence that were already well established. Recurrence rates were not commonly reported, though time from treatment and type of treatment were considered important factors. Patients receiving pharmacological treatment generally had lower recurrence rates, along with superior efficacy outcomes such as improvements in bleeding, discharge/leakage, or pain when compared to patients with non-pharmacological or no treatment. MPFF was the most investigated product among phlebotonics and had the highest quality of evidence, demonstrating that MPFF treatment improves the most important symptoms of HD. Among venoactive drugs, MPFF has shown some evidence supporting a benefit in the prevention of HD recurrence. However, further research on the risk of recurrence and the impact of treatments in the long term is needed.
Change history
28 February 2023
A peer-reviewed video abstract was retrospectively added to this publication.
References
Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). https://training.cochrane.org/handbook. Accessed Aug 2021.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008.
Abramowitz L, Benabderrahmane M, Pospait D, Philip J, Laouenan C. The prevalence of proctological symptoms amongst patients who see general practitioners in France. Eur J Gen Pract. 2014;20(4):301–6.
Conaghan P, Farouk R. Doppler-guided hemorrhoid artery ligation reduces the need for conventional hemorrhoid surgery in patients who fail rubber band ligation treatment. Dis Colon Rectum. 2009;52(1):127–30.
Godeberge P, Sheikh P, Zagriadskii E, et al. Hemorrhoidal disease and chronic venous insufficiency: concomitance or coincidence; results of the CHORUS study (Chronic venous and HemORrhoidal diseases evalUation and Scientific research). J Gastroenterol Hepatol. 2020;35(4):577–85.
Ince M, Ozdemir Y, Kucukerdonmez O, Levhi Akin M. Etiological risk factors of hemorroidal disease in youngs Anatolian. J Clin Investig. 2012;6(2):109–12.
Lin LH, Siu JJ, Liao PC, et al. Association of chronic obstructive pulmonary disease and hemorrhoids: a nationwide cohort study. Medicine (Baltimore). 2017;96(10):e6281.
Mascarello KC, Matijasevich A, da Silva dos Santos I, Silveira MF. Complicações puerperais precoces e tardias associadas à via de parto em uma coorte no Brasil. Rev Bras Epidemiol. 2018. https://doi.org/10.1590/1980-549720180010.
Peery AF, Sandler RS, Galanko JA, et al. Risk factors for hemorrhoids on screening colonoscopy. PLoS ONE. 2015;10(9):e0139100.
Peeters GM, Burton NW, Brown WJ. Associations between sitting time and a range of symptoms in mid-age women. Prev Med. 2013;56(2):135–41.
Ratto C, Donisi L, Parello A, Litta F, Doglietto GB. Evaluation of transanal hemorrhoidal dearterialization as a minimally invasive therapeutic approach to hemorrhoids. Dis Colon Rectum. 2010;53(5):803–11.
Yang JY, Peery AF, Lund JL, Pate V, Sandler RS. Burden and cost of outpatient hemorrhoids in the United States employer-insured population, 2014. Am J Gastroenterol. 2019;114(5):798–803.
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, et al. Meta-analysis of flavonoids for the treatment of haemorrhoids. Br J Surg. 2006;93(8):909–20.
Aziz Z, Huin WK, Badrul Hisham MD, Tang WL, Yaacob S. Efficacy and tolerability of micronized purified flavonoid fractions (MPFF) for haemorrhoids: a systematic review and meta-analysis. Complement Ther Med. 2018;39:49–55.
Perera N, Liolitsa D, Iype S, et al. Phlebotonics for haemorrhoids. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD004322.pub3.
Quijano CE, Abalos E. Conservative management of symptomatic and/or complicated haemorrhoids in pregnancy and the puerperium. Cochrane Database Syst Rev. 2005. https://doi.org/10.1002/14651858.CD004077.pub2.
Wanis KN, Emmerton-Coughlin HM, Coughlin S, Foley N, Vinden C. Systemic metronidazole may not reduce posthemorrhoidectomy pain: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2017;60(4):446–55.
Naldini G, Martellucci J, Rea R, et al. Tailored prolapse surgery for the treatment of haemorrhoids and obstructed defecation syndrome with a new dedicated device: TST STARR Plus. Int J Colorectal Dis. 2014;29(5):623–9.
Kendirci M, Şahiner İT, Şahiner Y, Güney G. Comparison of effects of vessel-sealing devices and conventional hemorrhoidectomy on postoperative pain and quality of life. Med Sci Monit. 2018;24:2173–9.
Sheikh P, Lohsiriwat V, Shelygin Y. Micronized purified flavonoid fraction in hemorrhoid disease: a systematic review and meta-analysis. Adv Ther. 2020;37(6):2792–812.
Godeberge P, Sheikh P, Lohsiriwat V, Jalife A, Shelygin Y. Micronized purified flavonoid fraction in the treatment of hemorrhoidal disease. J Comp Eff Res. 2021;10(10):801–13.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were sponsored by Servier.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Andreas Freitag, Kassandra Schaible, Monique Martin, and Pelin Yaltirik contributed to the concept and design of the study as well as drafting of the manuscript. April Camilla Roslani, Dong-Lin Ren, Philippe Godeberge, Parvez Sheikh, Robert Bandolon, and Varut Lohsiriwat contributed to the drafting of the manuscript. All authors read and approved the final version of the manuscript.
Disclosures
Pelin Yaltirik is an employee of Servier. Andreas Freitag was an employee of Evidera at the time the work was conducted and is now an employee of Cytel. Monique Martin and Kassandra Schaible are employees of Evidera. April Camilla Roslani, Dong-Lin Ren, Philippe Godeberge, Parvez Sheikh, Robert Bandolon, and Varut Lohsiriwat have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies; none of the authors performed any new studies involving human participants or animals.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lohsiriwat, V., Sheikh, P., Bandolon, R. et al. Recurrence Rates and Pharmacological Treatment for Hemorrhoidal Disease: A Systematic Review. Adv Ther 40, 117–132 (2023). https://doi.org/10.1007/s12325-022-02351-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02351-7