Abstract
Introduction
This study aimed to evaluate the cost-effectiveness of donafenib compared to sorafenib and lenvatinib as first-line treatments for patients with advanced hepatocellular carcinoma (HCC) in China.
Methods
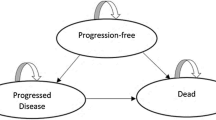
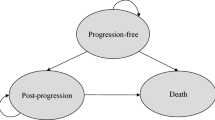
A partitioned survival model was developed to estimate the clinical and economic outcomes of donafenib, sorafenib, and lenvatinib for advanced HCC. The key clinical data of these targeted therapies were assessed through a network meta-analysis. The cost and health utilities were mainly collected from the literature. Quality-adjusted life years (QALYs), costs, and incremental cost-effectiveness ratios (ICER) were the primary outcomes. Model uncertainty was tested with one-way sensitivity analyses, scenario analyses, and probabilistic sensitivity analyses (PSA).
Results
For health outcomes, donafenib gained the highest QALYs among the three treatments, followed by lenvatinib and sorafenib (1.106, 0.999, and 0.915 QALYs, respectively). For cost, donafenib was the cheapest option, followed by sorafenib and lenvatinib ($42,116, $43,193, and $44,261). The PSA indicated that the probability of being cost-effective for donafenib was 86.98% and 93.56% when the willingness-to-pay thresholds were one and three times the gross domestic product per capita in China, respectively. The one-way sensitivity analyses and scenario analyses also found the results to be robust.
Conclusion
Compared to sorafenib and lenvatinib, donafenib was likely to be a cost-effective treatment with the highest QALYs and the lowest cost for patients with advanced HCC in China.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604.
Chen QF, Wu PH, Huang T, et al. Efficacy of treatment regimens for advanced hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(40): e17460.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73.
Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J Clin Oncol. 2021;39:3002–11.
Li X, Qiu M, Wang S, et al. A phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2020;85(3):593–604.
Chinese Society of Clinical Oncology Guidelines Working Committee. CSCO guidelines for the diagnosis and treatment of primary liver cancer. 2020th ed. Beijing: Medical Publishing House; 2020.
Yue X, Li Y, Wu J, Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Region Issues. 2021;24:1–5.
Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66.
Qin S, Li Q, Gu S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(7):559–68.
Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202.
El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–80.
Qin S, Kruger E, Tan SC, et al. Cost-effectiveness analysis of FOLFOX4 and sorafenib for the treatment of advanced hepatocellular carcinoma in China. Cost Eff Resour Alloc. 2018;16:29.
Ministry of Human Resources and Social Security, National Medical Security Administration. Notice of the Ministry of Human Resources and Social Security of the National Medical Insurance Administration on Printing and Distributing the "National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalog (2020). 2020. http://www.nhsa.gov.cn/art/2020/12/28/art_37_4220.html. Accessed 3 Mar 2022.
Ministry of Human Resources and Social Security, National Medical Security Administration. Notice of the Ministry of Human Resources and Social Security of the National Medical Insurance Administration on Printing and Distributing the "National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug Catalog (2021). 2021. http://www.gov.cn/zhengce/zhengceku/2021-12/03/content_5655651.htm. Accessed 3 Mar 2022.
Cai H, Zhang L, Li N, Zheng B, Liu M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: a cost-effectiveness analysis. J Comp Eff Res. 2020;9(8):553–62.
Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. 2018;6(1):124.
Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. 2021;4(2): e210037.
Wen F, Zheng H, Zhang P, et al. Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: a cost-effectiveness analysis in China and the United states. Liver Int. 2021;41(5):1097–104.
Kobayashi M, Kudo M, Izumi N, et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. 2019;54(6):558–70.
Kim JJ, McFarlane T, Tully S, Wong WWL. Lenvatinib versus sorafenib as first-line treatment of unresectable hepatocellular carcinoma: a cost-utility analysis. Oncologist. 2020;25(3):e512–9.
Guan H, Liu G, Xie F, Sheng Y, Shi L. Cost-effectiveness of osimertinib as a second-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer in China. Clin Ther. 2019;41(11):2308-20.e11.
Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–203.
Liu G, Hu S, Wu J, Dong Z, Li H. China guidelines for pharmacoeconomic evaluations. Beijing: China Market; 2020.
Wu B, Shi L. Frontline BRAF testing-guided treatment for advanced melanoma in the era of immunotherapies: a cost-utility analysis based on long-term survival data. JAMA Dermatol. 2020;156:1177.
Vogel A, Qin S, Kudo M, et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6(8):649–58.
Meng R, Cao Y, Zhou T, Hu H, Qiu Y. The cost effectiveness of donafenib compared with sorafenib for the first-line treatment of unresectable or metastatic hepatocellular carcinoma in China. Front Public Health. 2022;10: 794131.
Meyers BM, Vogel A, Marotta P, et al. The cost-effectiveness of lenvatinib in the treatment of advanced or unresectable hepatocellular carcinoma from a Canadian perspective. Can J Gastroenterol Hepatol. 2021;2021:8811018.
Saiyed M, Byrnes J, Srivastava T, Scuffham P, Downes M. Cost-effectiveness of lenvatinib compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma in Australia. Clin Drug Investig. 2020;40(12):1167–76.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China, grant no. 72104151. The journal's Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design: Haijing Guan, Sheng Han; Acquisition, analysis, or interpretation of data: Haijing Guan, Chunping Wang; Drafting of the manuscript: Haijing Guan, Chunping Wang; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: Haijing Guan, Chunping Wang; Administrative, technical, or material support: Zhigang Zhao, Sheng Han; Supervision: Sheng Han, Zhigang Zhao. All authors approved the final version of this article.
Disclosures
Haijing Guan, Chunping Wang, Zhigang Zhao, and Sheng Han all have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously published studies, modelling techniques and ethics committee approval is not required.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guan, H., Wang, C., Zhao, Z. et al. Cost-Effectiveness of Donafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma in China. Adv Ther 39, 3334–3346 (2022). https://doi.org/10.1007/s12325-022-02185-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02185-3