Abstract
Introduction
This study aimed to evaluate the cost-effectiveness of sintilimab plus bevacizumab versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma (HCC) in China to provide economic evidence to inform health decision making.
Methods
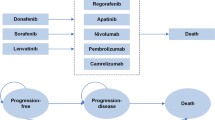
We performed an economic evaluation from the perspective of the Chinese healthcare system using a partitioned survival model with three mutually exclusive health states: progression free, post-progression, and death. Efficacy data were obtained from the ORIENT-32 clinical trial and extrapolated to the lifetime horizon. Cost and utility values were derived from published studies and online price databases. The primary outcomes of the model were quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs). Sensitivity analyses were carried out to verify the robustness of the model results.
Results
Compared with sorafenib, sintilimab plus bevacizumab incurred a higher lifetime cost ($33,766 vs. $23,294) and yielded more QALYs (1.428 vs. 0.928 QALYs). The ICER for sintilimab plus bevacizumab was $20,968/QALY and lower than the willingness-to-pay threshold of $33,592. The results of sensitivity analysis showed that ICER values were most sensitive to the subsequent treatment cost of the sorafenib group after progression and the price of bevacizumab. In the scenario analysis, the ICER was $4191/QALY when a 7.5 mg/kg dose of bevacizumab was applied in the model.
Conclusions
Compared with sorafenib, the sintilimab plus bevacizumab combination is likely to be a cost-effective option for patients with unresectable HCC in China.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Chinese Society of Clinical Oncology (CSCO). Guidelines for the diagnosis and treatment of primary liver cancer. 2020. http://www.amoydx.com/upfiles/reports/202103/1614847539702.pdf. Accessed 8 Jun 2021.
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010.
He Y, Zhang Z, He W, Hou J. Epidemiological characteristics and prognosis of hepatocellular carcinoma: a single center observational real world cohort study of 1302 cases. J Clin Hepatol. 2019;35(05):1002–7.
Cai Y, Xue M, Chen W, Hu M, Miao Z, Lan L, et al. Expenditure of hospital care on cancer in China, from 2011 to 2015. Chin J Cancer Res. 2017;29(3):253–62.
Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202.
Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564.
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–26.
Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22(7):977–90.
Liu GN, Hu SL, Wu JH, Wu J, Dong CH, Li HC. China guidelines for pharmacoeconomic evaluation (Chinese-English version). Beijing: China Market Press; 2020.
National Institute for health and care excellence. Bevacizumab (first-line), sorafenib (first- and second-line), sunitinib (second-line) and temsirolimus (first-line) for the treatment of advanced and/or metastatic renal cell carcinoma. https://www.nice.org.uk/guidance/ta178/resources/bevacizumab-firstline-sorafenib-first-and-secondline-sunitinib-secondline-and-temsirolimus-firstline-for-the-treatment-of-advanced-andor-metastatic-renal-cell-carcinoma-pdf-82598442394309. Accessed 10 Jun 2021.
Kim JJ, Mcfarlane T, Tully S, Wong WWL. Lenvatinib versus sorafenib as first-line treatment of unresectable hepatocellular carcinoma: a cost-utility analysis. Oncologist. 2020;25(3):e512–9.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90.
Saiyed M, Byrnes J, Srivastava T, Scufham P, Downes M. Cost-effectiveness of lenvatinib compared with sorafenib for the first-line treatment of advanced hepatocellular carcinoma in Australia. Clin Drug Investig. 2020;40(12):1167–76.
Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. 2021;4(2): e210037. https://doi.org/10.1001/jamanetworkopen.2021.0037.
Beijing Health Alliance Charitable Foundation. The patient assistance program of sintilimab. https://www.bjhacf.org/?p=3777. Accessed 3 Apr 2021.
Drug and medical service price. www.shuju.menet.com.cn. Accessed 5 Apr 2021.
Chiang JK, Kao YH. The impact of hospice care on survival and cost saving among patients with liver cancer: a national longitudinal population-based study in Taiwan. Support Care Cancer. 2015;23(4):1049–55.
Cai H, Zhang L, Li N, Zheng B, Liu M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: a cost-effectiveness analysis. J Comp Eff Res. 2020;9(8):553–62.
Zhang P, Wen F, Li Q. FOLFOX4 or sorafenib as the first-line treatments for advanced hepatocellular carcinoma: a cost-effectiveness analysis. Dig Liver Dis. 2016;48(12):1492–7.
Zhang P, Yang Y, Wen F, He X, Tang R, Du Z, et al. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27(7):853–9.
Kobayashi M, Kudo M, Izumi N, Kaneko SC, Azuma M, Copher R, et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. 2019;54(6):558–70.
Wen F, Zheng H, Zhang P, Liao W, Zhou K, Li Q. Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: a cost-effectiveness analysis in China and the United States. Liver Int. 2021;41(5):1097–104.
Granito A, Marinelli S, Negrini G, Menetti S, Benevento F, Bolondi L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Therap Adv Gastroenterol. 2016;9(2):240–9.
Acknowledgements
Funding
This study, as well as the journal’s Rapid Service Fees, was funded by Innovent Biologics, Inc., and supported by the Fundamental Research Funds for the Central Universities (no. 2632022PY03). The funders were not involved in the study design, collection, analysis, interpretation of data, and the writing and the publication of this work.
Medical Writing and Editorial Assistance
Language editing support was provided by the “Nature research editing service” of Springer Nature and funded by Innovent Biologics, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Ting Zhou, Hong Chao Li and Aixia Ma conceived and designed this work. Ting Zhou, Aixia Ma, Yingdan Cao acquired the data. Ting Zhou, Hong Chao Li, Xintian Wang, Zijing Wang and Lan Yang analyzed the data. Hong Chao Li and Aixia Ma supervised the study and acquired funding. Ting Zhou, Xintian Wang, Yingdan Cao, Lan Yang prepared the original manuscript. Hong Chao Li, Aixia Ma revised and edited the manuscript. All authors reviewed, read and approved the final version of the manuscript.
Disclosures
Ting Zhou, Yingdan Cao, Xintian Wang, Lan Yang, Zijing Wang, Aixia Ma and Hong Chao Li declare no other potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Compliance with Ethics Guidelines
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent from the patients was not required in this study because the research data is publicly available. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data analyzed during this work were included in this article.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, T., Cao, Y., Wang, X. et al. Economic Evaluation of Sintilimab Plus Bevacizumab Versus Sorafenib as a First-line Treatment for Unresectable Hepatocellular Carcinoma. Adv Ther 39, 2165–2177 (2022). https://doi.org/10.1007/s12325-022-02079-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02079-4