Abstract
Introduction
There is limited real-world evidence on the treatments for atopic dermatitis (AD) since dupilumab was approved in 2017. The objective of this study was to assess market share of drugs commonly prescribed for the treatment of AD and describe treatment patterns in patients diagnosed with AD.
Methods
This was a retrospective, observational study in adult patients with an ICD-10 diagnosis of AD between 2017 and 2019 using insurance claims data in the US population.
Results
Market share cohorts consisted of 75,794 (2017) and 89,618 (2018) patients. Treatment patterns cohort had 68,588 patients with 63.56% female, mean (SD) age 43.54 (15.96) years, and mean (SD) Quan CCI 0.31 (0.85). Topicals had two-thirds market share by prescription volume (2017 = 65.56%; 2018 = 63.63%). Corticosteroids were the most prescribed topical (2017 = 71.94%; 2018 = 72.04%) and systemic (2017 = 30.59%; 2018 = 30.23%) drug class. Dupilumab had the highest medication adherence (proportion of days covered [PDC] ≥ 80%; 60.74%) and persistence (17.39%), lowest discontinuation rate (23.32%), and longest mean (SD) days on therapy 148.20 (101.77).
Conclusion
Topicals are the primary treatment for patients with AD, even though systemic users have higher medication adherence (PDC). Systemics provide a treatment alternative to topicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a paucity of real-world data on market share and treatments for atopic dermatitis (AD) since dupilumab approval in 2017 in the USA |
The study aimed to evaluate the market share of drugs commonly prescribed for the treatment of AD and describe treatment patterns in patients diagnosed with AD using insurance claims data in the US population |
What was learned from the study? |
Corticosteroids, both topical and systemic, had the largest market share in the treatment of AD in 2017 and 2018 |
Topicals continue to be the primary treatment option for patients with AD, even though systemics have higher adherence and persistence, lower discontinuation rates, and a longer time on continuous therapy |
Systemics offer a treatment alternative to topicals in the long-term management of AD |
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease. The disease is heterogeneous in presentation and characterized by pruritus and recurrent eczematous lesions caused by skin barrier dysfunction and dysregulation of the immune system [1,2,3]. The prevalence of AD among adults in the US population is estimated to be approximately 7.0% [4]. AD impairs patient’s quality of life and is associated with several comorbidities including sleep disturbance, allergic comorbidities (i.e., asthma, allergic rhinitis), and mental health disorders (i.e., anxiety, depression) [5,6,7,8].
Recent guidelines from the Joint Task Force (JTF) and the American Academy of Dermatology (AAD) recommend use of non-pharmacologic and pharmacologic interventions in the management of AD [9]. Non-pharmacologic interventions include good skin care through bathing, regular use of moisturizers, and wet-wrap therapy [9]. Pharmacologic interventions include both approved and off-label use of topical and systemic therapies to treat moderate-to-severe AD. Topical therapies commonly used include corticosteroids (TCS), calcineurin inhibitors (TCI), and phosphodiesterase-4 inhibitors (PDE-4i). Conventional systemic therapies consist of corticosteroids (SCS), immunosuppressants (SIS), antihistamines, and biologics [9]. Topical therapy is considered first-line therapy and is especially effective in treating patients diagnosed with AD who fail to respond to good skin care and regular use of emollients [9, 10]. The JTF and AAD recommend prescribing systemic therapy in the subset of patients that fail topical therapy and/or phototherapy [9, 11].
Until recently, systemic therapies approved to treat moderate-to-severe AD were limited to corticosteroids; however, in 2017 the Food and Drug Administration (FDA) approved dupilumab, an interleukin-4/13 inhibitor (IL4/13i) fully human monoclonal antibody [12]. Since 2017 there have been several new classes of systemic therapies entering early and late-phase clinical development for moderate-to-severe AD including oral Janus kinase inhibitors (JAKi), interleukin-13 inhibitors, interleukin-31 inhibitor, and OX40 (CD134) [1, 13,14,15,16]. There is a paucity of real-world data in the US on patients with moderate-to-severe AD following the approval of dupilumab. Given the heterogeneity of the disease, it is important to understand the characteristics and treatment patterns in this patient population with more recent data [17,18,19]. The objectives of this study were to evaluate the market share of drugs commonly prescribed for the treatment of AD and describe treatment patterns in patients diagnosed with AD using insurance claims data in the US population.
Methods
Study Design and Data Sources
This was a retrospective, observational study conducted using IBM MarketScan Research Databases® adjudicated US insurance claims. This database comprises both the Commercial Claims and Encounters (Commercial) and the Medicare Supplemental and Coordination of Benefits (Medicare Supplemental) Databases [20, 21]. MarketScan contains information on inpatient medical, outpatient medical, and outpatient prescription drug claims of employees and their dependents covered under a variety of fee-for-service and capitated health plans or Medicare supplemental insurance. All study data were accessed with protocols compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations. Because all databases used in the study are fully deidentified and compliant with the HIPAA, this study was exempted from Institutional Review Board approval.
Selection Criteria
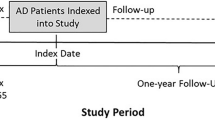
Selection criteria were used to define three patient cohorts: two market share cohorts (2017 and 2018) and one treatment patterns cohort. For the market share cohorts, patients were required to have at least one AD diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 691.8, or Tenth Revision [ICD-10-CM] diagnosis codes L20.0, L20.81, L20.82, L20.83, L20.84, L20.89, or L20.9) between January 1, 2017, and December 31, 2019. Patients were aged ≥ 18 years as of the first observed AD diagnosis and continuously enrolled in medical and pharmacy benefits for 12 months before and after the AD diagnosis. The treatment patterns cohort was a subset of the market share cohorts and was defined based on additional selection criteria of having at least one National Drug Code (NDC) medication code for one of the selected drug classes used for the treatment of AD from January 1, 2017, to December 31, 2019. The index date was defined as the date of the first observed prescription claim for AD during the study period. Patients included in the cohort had no National Drug Code (NDC) medication code for the index drug classes in the 12 months prior to the index date and continuous enrollment both 12 months before (baseline) and after (follow-up) the index date. The baseline period was used to describe demographic and clinical characteristics of patients with AD and confirm true initiation of treatment for AD in the follow-up period. Patients were then followed from the index date for 12 months to evaluate treatment patterns (Figure S1).
Study Measurements and Outcomes
Market shares were measured cross-sectionally in 2017 and 2018 and included all drug classes associated with the treatment of AD. Demographic and clinical variables collected at baseline included age, sex, primary payer, health plan, geographic region, employment status, length of follow-up, Quan Charlson Comorbidity Index (CCI), any prior diagnosis of AD, prior dermatology office visits, and comorbidities. Concomitant medications were collected at both baseline and follow-up to evaluate burden and drug sparing. Treatment patterns among patients with AD were assessed during the follow-up and based on concomitant use, refills, adherence, persistence, and discontinuations. Drug classes included in the assessment of the treatment patterns were a subset of the drug classes in the market share analyses and based on the most filled drugs to treat AD. Both topical (antihistamines, TCI, TCS, PDE-4i, retinoids, vitamin D3 analogues) and systemic (antihistamines, SCS, IgEi, IL4/13i, immune globulin, SIS, IFN-gamma, JAKi, PDE-4i, retinoids) drug classes were included in the analysis.
Medication adherence was measured using two different methods, proportion of days covered (PDC) and medication possession ratio (MPR). PDC was defined as the sum of the number of days with drug during the follow-up divided by the number of days available in the patient’s observation period. The proportion of patients with PDC ≥ 80% was reported by drug class. The measure was capped at 1.0 adjusted for overlapped days of drug supply. MPR was defined as the total days’ supply divided by number of days between the first and last prescription claim, plus the days’ supply for the last prescription claim among patients with ≥ 2 prescription claims. The proportion of patients with MPR ≥ 80% was reported by drug class.
Persistence was measured as the proportion of patients continuously treated during the 12-month follow-up, adjusting for any overlapping days’ supply (≤ 60-day gap between prescription claims during 12-month follow-up period). Discontinuation was defined as the number of patients who were not using any AD drug for > 60 consecutive days. Number of patients who discontinued and days to discontinuation from the index date were reported and further stratified by drug class.
Statistical Analysis
Statistical analyses were performed using Instant Health Data, a SaaS-based RWE analytics platform (BHE, Boston, MA, USA). Descriptive statistics were conducted using R, Version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were presented as the count and proportion of patients in each category; continuous variables were summarized by mean and standard deviation (SD).
Results
Market Share
Patient demographic and clinical characteristics were similar between cohorts (Table 1), so only baseline data from the 2018 cohort (n = 89,618) are reported. Approximately two-thirds were female (63.91%) with a mean (SD) age of 44.64 (16.25) years. The largest share of patients resided in the southern region of the US (38.40%) and over half were using a preferred provider organization (PPO) health plan (54.26%). The mean (SD) Quan CCI was 0.06 (0.28).
There were 220,857 prescriptions filled for medications associated with AD in 2017 followed by an increase in prescription volume in 2018 to 268,581 (Table 2). Topicals (2017 = 65.56%; 2018 = 63.63%) had the highest market share compared to oral medications (2017 = 31.03%; 2018 = 28.74%) and injectables (2017 = 3.41%; 2018 = 7.63%). Among drug classes, corticosteroids had the largest market share in 2017 (TCS = 60.60%; SCS = 19.22%) and 2018 (TCS = 57.44%; SCS = 18.06%). Dupilumab showed a nearly three-fold increase in market share in 2018 (6.68%) compared to 2017 (2.39%). The market share of oral medications decreased in 2018 (28.74%) compared to 2017 (31.03%), while there was a noticeable increase in the use of injectables between 2017 (3.41%) and 2018 (7.63%).
Nearly three-quarters of patients were prescribed topicals (2017 = 73.84%; 2018 = 74.58%) and over one-third were taking oral medications (2017 = 35.80%; 2018 = 35.18%). Injectables were prescribed in < 5% of patients in 2017 (2.00%) and 2018 (3.29%). Like market share, the largest proportion of patients was on corticosteroids in 2017 (TCS = 71.94%; SCS = 30.59%) and 2018 (TCS = 72.04%; SCS = 30.23%). There was an increase in the number of patients prescribed each drug class between 2017 and 2018 except for SIS and IFN-gamma. However, the number of patients using dupilumab (2017 = 1148; 2018 = 2533) and topical PDE-4i (2017 = 2169; 2018 = 4395) in 2018 more than doubled from the previous year.
Treatment Patterns
There were 622,061 patients (i.e., starting population) in MarketScan with an ICD-9/10 diagnosis code for AD during the study period. After applying the selection criteria, the final cohort consisted of 11.03% (n = 68,588) of the starting population (Fig. 1). The mean (SD) age of the cohort was 43.54 (15.96) years with most patients in either the 18–40 (42.38%) or 41–60 (44.47%) year old age groups. The patients were mainly female (63.56%); the largest proportion of patients resided in the South (38.00%). Most patients had PPO health plans (54.82%) and approximately three-quarters were active full-time employees. Patients had a mean (SD) Quan CCI of 0.31 (0.85) and a mean (SD) length of follow-up of 24.39 (7.73) months. Approximately 19.00% of the patients had a diagnosis of AD at baseline, and nearly one-quarter (23.94%) visited a dermatologist during that same period (Table 1).
The most common AD-associated comorbidities were anxiety (13.42%), allergic rhinitis (10.75%), depression (10.08%), and asthma (6.54%). The most frequent general comorbidities were respiratory tract infections (22.77%), hypertension (22.41%), and hyperlipidemia (20.70%), as indicated in Table S1 in the electronic supplementary material. The most common concomitant drug classes at baseline and follow-up included pain medications, antibiotics, and medications treating asthma, anxiety, sleep disorders, and allergies. There was a noticeable increase in concomitant therapy burden across all drug classes after the initiation of treatment for AD (Table S2 in the electronic supplementary material).
Treatment patterns in the 12-month follow-up period are presented in Table 3. The mean (SD) number of refills was higher for oral medications (1.17 [0.48]) and injectables (1.28 [0.68]) than topicals (1.07 [0.31]). Among systemics, refills were highest among SIS (1.32 [0.66]) and dupilumab (1.28 [0.68]). Patients using topicals reported higher discontinuation rates (96.55%) compared to oral medications (92.98%) and injectables (29.38%). Discontinuation rates were lowest among patients taking dupilumab (23.32%), systemic PDE-4i (36.07%), and SIS (50.10%). The time to discontinuation in mean (SD) days for injectables (149.59 [93.79]) was longer than for oral medications (53.89 [76.51]) and topicals (48.66 [56.96]). Systemic PDE-4i (194.95 [84.21]), dupilumab (174.68 [82.37]), and SIS (154.69 [88.82]) had the longest time to discontinuation among drug classes.
Patients using injectables had a higher percentage of adherence as calculated using PDC ≥ 80% (55.69%) and MPR ≥ 80% (75.75%) compared to oral medications (PDC ≥ 80% = 1.98%; MPR ≥ 80% = 15.32%) and topicals (PDC ≥ 80% = 1.19%; MPR ≥ 80% = 13.70%). Medication adherence was highest among patients treated with dupilumab (PDC ≥ 80% = 60.74%; MPR ≥ 80% = 77.48%), systemic PDE-4i (PDC ≥ 80% = 42.62%; MPR ≥ 80% = 68.57%), and SIS (PDC ≥ 80% = 35.52%; MPR ≥ 80% = 73.39%). Injectable users also had higher persistence (15.88%) compared to oral medications (1.06%) and topical (0.85%) users. Like adherence, medication persistency was highest among dupilumab (17.39%), SIS (9.65%), and systemic PDE-4i (6.56%) users.
Length of therapy (mean [SD]) was only 24.36 (41.84) days for oral administration, 45.72 (41.58) days for topical applications, and a high of 137.31 (103.97) mean days for injections (Fig. 2). Patients on dupilumab had the longest time on therapy at 148.20 (101.77) days followed by SIS (110.53 [92.94]) and systemic PDE-4i (96.18 [80.51]). Patients taking all other drug classes were on continuous therapy for < 45 days (14.69–44.77 [21.64–59.51]). Continuous therapy stratified by monotherapy and combination therapy did not yield much difference in mean (SD) days on therapy across the drug classes, except for patients using injectables as monotherapy. Patients prescribed monotherapy dupilumab were continuously treated for 257.79 (94.48) days followed by SIS (154.70 [126.41]) and systemic PDE-4i (120.00 [79.37]).
Persistence measured as length of therapya in patients with atopic dermatitis. aLength of therapy was calculated based on the number of days of continuous therapy from the index date until the end of the follow-up, allowing for the 60-day gap between fills, reported by drug class. IL4/13i interleukin-4/13 inhibitor; PDE-4 phosphodiesterase-4 inhibitors; SCS systemic corticosteroids; SIS systemic immunosuppressants; TCI topical calcineurin inhibitors; TCS topical corticosteroids. Error bars indicate standard error of the mean
Discussion
This study aimed to assess market share and evaluate current trends in treatment patterns among patients with AD using MarketScan. Topicals accounted for approximately two-thirds market share, whereas oral medications accounted for about one-third of all prescriptions filled. Of all the drug classes related to the treatment of AD, corticosteroids had the largest market share with most patients on TCS (2017 = 71.94%; 2018 = 72.04%) or SCS (2017 = 30.59%; 2018 = 30.23%). The use of injectables more than doubled in market share in 2018 compared to 2017, driven mainly by volume growth of dupilumab. The plausible differences in the market share data might be attributed to the way these drug classes are used to treat different disease severities. Topicals, SCS, and antihistamines are used intermittently, whereas SIS and dupilumab are used for long-term management of patients with more severe AD.
Dupilumab ranked sixth in prescription volume (2.39%) in just its first 9 months on the market in 2017 and fourth in market share (6.68%) in its first full year on the market in 2018. During this same period, the number of patients prescribed dupilumab more than doubled from 1148 in 2017 to 2533 in 2018. Overall, there was a modest shift in prescription volume toward injectables, as both oral medications and topicals saw decreases in market share in 2018. The rapid increase in both the volume of prescriptions and number of patients using injectables may suggest an unmet need in AD including the wrong choice of topicals and lack of combination treatments and targeted treatments [22]. Given this, conventional therapies may not have been efficacious or well tolerated in long-term management of the disease. Despite this shift toward injectables in 2018, the number of patients prescribed topicals increased modestly, potentially indicating more intermittent use of topicals and, therefore, lower refill rates as more patients were prescribed injectables. The decrease in antihistamines and SCS may indicate better disease control with injectables. The number of patients using SIS was low with decreasing market share in 2018, possibly due to low efficacy or side effects of these drugs.
Overall, the findings were very consistent across all treatment pattern measures, especially for oral medications and injectables. For example, dupilumab, SIS, and systemic PDE-4i had the highest adherence and persistence, the lowest rates of discontinuation, and the longest time-to-treatment discontinuation and duration of continuous use among all topical and systemic drug classes. Dupilumab and SIS were used the longest as monotherapy versus combination therapy when compared to the other drug classes. Lower persistence for SCS and topicals reported in this study could suggest intermittent use due to milder disease and limited supply of these drugs. Injectables had a higher number of mean refills (1.28), lower discontinuation rate (29.38%), and higher mean days to discontinuation (149.59) compared to topicals and oral medications. Injectables also showed higher adherence for both PDC (55.69%) and MPR (75.75%) as well as higher persistence of 15.88%. Lower adherence PDC and MPR rates among topical and SCS users could be driven by the intent of the healthcare provider (HCP) to not prescribe topicals or SCS as a long-term treatment option. For example, a high-potency TCS might be prescribed for short duration before transitioning to a lower potency TCS or steroid-sparing topical. Other factors contributing to the adherence PDC and MPR and persistence rates may include loss of efficacy, side effects, symptom improvement, and waxing and waning of disease because of the severity of AD.
These study findings are consistent with previously published results using another claims database, IQVIA Health Plan, in the US population [18]. In that study, dupilumab showed higher persistence of 75.0% compared to SCS (27.8%) and SIS (1.6%) [18]. In this study, dupilumab showed higher persistence rates (17.39%) compared to SCS (0.20%) and SIS (9.65%). Many factors may contribute to the big disparity in persistency rates between this study and the Eichenfield et al. [18] study including study design, data source, and sample size. Eichenfield et al. [18] only included patients with moderate-to-severe AD treated with systemics or phototherapy (n = 1980) with 6-month baseline and follow-up periods.
Limitations
This study has several limitations. Results are not generalizable beyond commercially insured patients because claims data are limited to private health insurance and Medicare supplemental coverage. Furthermore, the study cohort is not representative of all health insurance, including lack of insurance. Other limitations include potential misclassification bias due to the inability to confirm AD diagnoses by medical record review, causing incorrect coding, data entry errors, or incomplete data. Market share could be overestimated, as prescription claims cannot be linked to specific diagnoses. Disease severity is an important factor when evaluating treatment patterns; however, the severity of AD was not available in MarketScan. Lastly, medication adherence is not easily measured in claims data using PDC and MPR, as these indirect measurement methods cannot adjust for factors like prescriber intent, inaccessibility, and patient trust in their HCP. Moreover, adherence PDC and MPR do not imply the patient elected to stop treatment against medical advice. The intent of the prescriber could be short-term use to address an AD flare. Adherence PDC and MPR assume the rates of prescription refills are in concordance with medication-taking behavior and therefore can only be approximated in a claims-based, retrospective, observational study. The study is descriptive in nature and therefore plausible extrapolations for MPR apart from patient adherence, such as medication hoarding, illegal medication resale, and medication use by persons other than the intended patient were not considered.
Conclusion
This study showed corticosteroids (topical and systemic) had the largest market share in the treatment of AD and rapid growth in prescription volume of injectables. Topicals continue to be the primary treatment option for patients with AD, even though systemics have higher adherence (PDC and MPR) and persistence, lower discontinuation rates, and a longer time for continuous therapy. The lower proportion of patients on systemic therapy and the higher adherence (PDC and MPR) and persistency rates among systemic users underscore the requisite for prescribers to better understand the current treatment paradigm for patients with AD. Future studies could focus on generating real-world evidence on the clinical benefits and improved quality of life that advanced therapies may provide patients with AD.
References
Boguniewicz M, Alexis AF, Beck LA, Block J, Eichenfield LF, Fonacier L, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5(6):1519–31.
Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22.
Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–51.
Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605.
Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–52.
Simpson EL, Bieber T, Eckert L, Wu R, Ardeleanu M, Graham NM, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–8.
Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137(1):18–25.
Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93(5):222–4.
Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–57.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–49.
FDA. FDA Product Label. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf.
Thaci D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52.
Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–21.
Newsom M, Bashyam AM, Balogh EA, Feldman SR, Strowd LC. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80(11):1041–52.
Dattola A, Bennardo L, Silvestri M, Nistico SP. What’s new in the treatment of atopic dermatitis? Dermatol Ther. 2019;32(2):e2787.
Igarashi A, Fujita H, Arima K, Inoue T, Dorey J, Fukushima A, et al. Health-care resource use and current treatment of adult atopic dermatitis patients in Japan: a retrospective claims database analysis. J Dermatol. 2019;46(8):652–61.
Eichenfield LF, DiBonaventura M, Xenakis J, Lafeuille MH, Duh MS, Fakih I, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the united states: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10(4):791–806.
Silverberg JI, Guttman-Yassky E, Gadkari A, Kuznik A, Mallya UG, Mastey V, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2020;2020:1.
IBM. What IBM MarketScan Research Databases can do for you. 2020. https://www.ibm.com/us-en/marketplace/marketscan-research-databases.
IBM. What IBM Explorys Database & Analytic Tools can do for you. 2020. https://www.ibm.com/us-en/marketplace/explorys-ehr-data-analysis-tools.
Langenbruch A, Radtke M, Franzke N, Ring J, Foelster-Holst R, Augustin M. Quality of health care of atopic eczema in Germany: results of the national health care study AtopicHealth. J Eur Acad Dermatol Venereol. 2014;28(6):719–26.
Acknowledgements
Funding
This research and Rapid Service and Open Access Fees were funded by Eli Lilly and Company, USA.
Medical Writing and Editorial Assistance
The authors appreciate Uma Jyothi Kommoju, PhD, an employee of Eli Lilly Services India Private Limited for providing medical writing support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Christian Fenske contributed to the design, acquisition, analysis, and interpretation of the data, as well as drafting and critical revision of the manuscript. Natalie Boytsov contributed to the analysis and interpretation of the data and critical revision of the manuscript. Jiaying Guo contributed to the analysis and interpretation of the data and critical revision of the manuscript. Zach Dawson contributed to the design, interpretation of the data and critical revision of the manuscript.
Disclosures
Christian Fenske, Jiaying Guo and Zach Dawson are employees and/or minor stockholders of Eli Lilly and Company. Natalie Boytsov is a former employee of Eli Lilly and Company, Indianapolis.
Compliance with Ethics Guidelines
All study data were accessed with protocols compliant with United States patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations. Because all databases used in the study are fully deidentified and compliant with the HIPAA, this study was exempted from Institutional Review Board approval. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The data that support the findings of this study were provided by IBM. The datasets generated during and/or analyzed during the current study are not publicly available due to restrictions that apply to the availability of these data, which were used under license for this study. Requests may be sent to IBM for more information on data availability and licensing.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fenske, C., Boytsov, N., Guo, J. et al. Prescription Market Share and Treatment Patterns in Atopic Dermatitis: A Retrospective Observational Study Using US Insurance Claims. Adv Ther 39, 2052–2064 (2022). https://doi.org/10.1007/s12325-022-02071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02071-y