Abstract
Introduction
The canonical isocitrate dehydrogenase 1 R132 mutation (IDH1 R132) is the most frequent mutation among IDH-mutated gliomas. Non-canonical IDH1 mutations or IDH2 mutations are unusual and their clinical and biological role is still unclear.
Methods
We performed a systematic review and meta-analysis to assess the clinical role of IDH non-canonical mutations.
Results
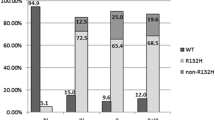
Overall, we selected 13 of 3513 studies reporting data of 4007 patients with a diagnosis of grade 2 and grade 3 glioma including 3091 patients with a molecularly proven IDH1 or IDH2 mutation. Patients with non-canonical IDH1 mutations were younger and presented a higher DNA methylation level as compared to those with canonical IDH1 R132H alteration. The overall incidence of non-canonical IDH1 mutations was 7.9% (95% CI 5.4–10.7%) in patients with IDH-mutated gliomas. There was no statistical difference in terms of incidence between patients with grade 2 or grade 3 glioma. Patients with non-canonical IDH mutations had a lower rate of 1p19q codeletion (risk difference 31%, 95% CI 23–38%) and presented a significantly prolonged survival (pooled HR 0.47, 95% CI 0.28–0.81) as compared to those with IDH1 R132H mutation.
Conclusion
Non-canonical IDH1 mutations occur in 7.9% of IDH-mutated gliomas and identify a specific subgroup of patients with an improved survival despite a lower rate of 1p19q codeletion. Data about the type of IDH mutation should be collected in clinical practice and within interventional trials as this could be a critical variable for improved stratification and selection of patients.





Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–100. https://doi.org/10.1093/neuonc/noz150.
Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–86. https://doi.org/10.1038/s41571-020-00447-z.
Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–98. https://doi.org/10.1056/NEJMoa1402121.
Mair MJ, Geurts M, van den Bent MJ, Berghoff AS. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat Rev. 2021;92: 102124. https://doi.org/10.1016/j.ctrv.2020.102124.
Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4. https://doi.org/10.1200/jco.2009.21.9832.
Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. https://doi.org/10.1056/NEJMoa0808710.
Wang HY, Tang K, Liang TY, et al. The comparison of clinical and biological characteristics between IDH1 and IDH2 mutations in gliomas. J Exp Clin Cancer Res. 2016;35:86. https://doi.org/10.1186/s13046-016-0362-7.
Tesileanu CMS, Vallentgoed WR, Sanson M, et al. Non-IDH1-R132H IDH1/2 mutations are associated with increased DNA methylation and improved survival in astrocytomas, compared to IDH1-R132H mutations. Acta Neuropathol. 2021;141(6):945–57. https://doi.org/10.1007/s00401-021-02291-6.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Poetsch L, Bronnimann C, Loiseau H, et al. Characteristics of IDH-mutant gliomas with non-canonical IDH mutation. J Neurooncol. 2021;151(2):279–86. https://doi.org/10.1007/s11060-020-03662-x.
Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120(6):719–29. https://doi.org/10.1007/s00401-010-0777-8.
Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–74. https://doi.org/10.1007/s00401-009-0561-9.
Gravendeel LA, Kloosterhof NK, Bralten LB, et al. Segregation of non-pR132H mutations in IDH1 in distinct molecular subtypes of glioma. Hum Mutat. 2010;31(3):E1186–99. https://doi.org/10.1002/humu.21201.
Franceschi E, De Biase D, Di Nunno V, et al. IDH1 non-canonical mutations and survival in patients with glioma. Diagnostics (Basel). 2021. https://doi.org/10.3390/diagnostics11020342.
Chen N, Yu T, Gong J, et al. IDH1/2 gene hotspot mutations in central nervous system tumours: analysis of 922 Chinese patients. Pathology. 2016;48(7):675–83. https://doi.org/10.1016/j.pathol.2016.07.010.
Camelo-Piragua S, Jansen M, Ganguly A, et al. A sensitive and specific diagnostic panel to distinguish diffuse astrocytoma from astrocytosis: chromosome 7 gain with mutant isocitrate dehydrogenase 1 and p53. J Neuropathol Exp Neurol. 2011;70(2):110–5. https://doi.org/10.1097/NEN.0b013e31820565f9.
Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: a phase III trial of radiation versus radiation plus procarbazine, lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020;38(29):3407–17. https://doi.org/10.1200/jco.19.02983.
Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. https://doi.org/10.1007/s00401-008-0455-2.
Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–63. https://doi.org/10.1016/j.cell.2015.12.028.
van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053–22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–53. https://doi.org/10.1016/s0140-6736(17)31442-3.
van den Bent MJ, Klein M, Smits M, et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): a randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018;19(9):1170–9. https://doi.org/10.1016/s1470-2045(18)30362-0.
Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51. https://doi.org/10.1093/neuonc/noab106.
Tesileanu CMS, van den Bent MJ, Sanson M, et al. Prognostic significance of genome-wide DNA methylation profiles within the randomized, phase 3, EORTC CATNON trial on non-1p/19q deleted anaplastic glioma. Neuro Oncol. 2021;23(9):1547–59. https://doi.org/10.1093/neuonc/noab088.
Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580–9. https://doi.org/10.1038/s41416-020-0814-x.
Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. https://doi.org/10.1186/1471-2288-14-45.
Visani M, de Biase D, Marucci G, et al. Expression of 19 microRNAs in glioblastoma and comparison with other brain neoplasia of grades I-III. Mol Oncol. 2014;8(2):417–30. https://doi.org/10.1016/j.molonc.2013.12.010.
Ferreyra Vega S, Olsson Bontell T, Corell A, Smits A, Jakola AS, Carén H. DNA methylation profiling for molecular classification of adult diffuse lower-grade gliomas. Clin Epigenet. 2021;13(1):102. https://doi.org/10.1186/s13148-021-01085-7.
Pirozzi CJ, Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol. 2021. https://doi.org/10.1038/s41571-021-00521-0.
Gatto L, Franceschi E, Tosoni A, et al. IDH inhibitors and beyond: the cornerstone of targeted glioma treatment. Mol Diagn Ther. 2021. https://doi.org/10.1007/s40291-021-00537-3.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contribution
EF, VDN, AAB, conceptualization. EF, VDN, AT, LG, IM, SB, DA, writing, software and data curation. VDN, EF, statistical analyses. AAB and RL, supervision, visualization and investigation. All authors helped in review and editing.
Disclosures
All authors (Vincenzo Di Nunno, Enrico Franceschi, Alicia Tosoni, Lidia Gatto, Ilaria Maggio, Daniele Angelini, Stefania Bartolini, Raffaele Lodi, Alba Ariela Brandes) all have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Nunno, V., Franceschi, E., Tosoni, A. et al. Clinical and Molecular Features of Patients with Gliomas Harboring IDH1 Non-canonical Mutations: A Systematic Review and Meta-Analysis. Adv Ther 39, 165–177 (2022). https://doi.org/10.1007/s12325-021-01977-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01977-3