Abstract
Introduction
Cigarette smoking remains a substantial public health problem. Nicotine replacement therapy (NRT) is an effective treatment that increases the success of a quit attempt. There are different NRT formats with no difference in efficacy, but their pharmaceutical form or route of administration may translate into individual preferences. A novel prototype mini lozenge was developed to offer smokers a new NRT option to aid in their quit attempt. Two studies were conducted to characterize the pharmacokinetic parameters and to evaluate its bioequivalence to a commercially available nicotine mini lozenge.
Methods
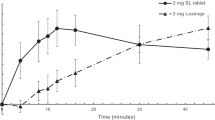
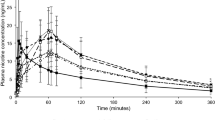
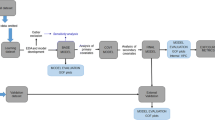
Two randomized, open-label, crossover studies were conducted to evaluate either the 2 or 4 mg dose level. Heavy smokers in otherwise good health were randomly assigned to one of two treatment sequences: the prototype mini lozenge followed by a commercially available mini lozenge, or the converse. After a 5 to 7 day washout period, subjects crossed over to receive the other study treatment. Blood sampling occurred pre- and post-dose nicotine and was assessed using a validated solid-phase extraction with ultra-high-performance liquid chromatography and tandem mass spectrometry. The primary endpoint was bioequivalence as determined by maximal plasma nicotine concentration (Cmax) and the extent of nicotine absorption (AUC0–t and AUC0–∞). The secondary endpoints included the time to Cmax (Tmax), half-life, the elimination constant (Kel), and safety.
Results
The prototype mini lozenge was bioequivalent to the commercially available mini lozenge, with no significant difference in Cmax, AUC0–t, or AUC0–∞ or any of the secondary outcomes. The most common treatment-emergent adverse event was throat irritation, of which all cases were mild in severity. There were no serious adverse events.
Conclusion
The prototype mini lozenge is bioequivalent to a commercially available mini lozenge and may provide smokers with a new oral NRT option to aid in smoking cessation and of tobacco dependence through the relief of nicotine withdrawal symptoms, including cravings.


Similar content being viewed by others
References
World Health Organization. Tobacco. May 27, 2020. https://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed May 10, 2021.
Christensen CH, Rostron B, Cosgrove C, et al. Association of cigarette, cigar, and pipe use with mortality risk in the US population. JAMA Intern Med. 2018;178(4):469–76.
Adhikari B, Kahende J, Malarcher A, Pechacek T, Tong V. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Weekly. 2008;57(45):1226–8.
Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–303.
Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60.
Russell MA. Nicotine replacement: the role of blood nicotine levels, their rate of change, and nicotine tolerance. Prog Clin Biol Res. 1988;261:63–94.
Svensson CK. Clinical pharmacokinetics of nicotine. Clin Pharmacokinet. 1987;12(1):30–40.
Flowers L. Nicotine replacement therapy. Am J Psychiatry Residents’ J. 2016;11(6):4–7.
Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–64.
Borland R, Partos TR, Yong HH, Cummings KM, Hyland A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction. 2012;107(3):673–82.
Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update. Rockville: U.S. Department of Health and Human Services; 2008.
Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5:CD000146.
Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2019;4:CD013308.
Hollander and Wolf. Nonparametric statistical methods. 2nd ed. Hoboken: Wiley; 1999.
Shiffman S, Dresler CM, Hajek P, Gilburt SJA, Targett DA, Strahs KR. Efficacy of a nicotine lozenge for smoking cessation. Arch Intern Med. 2002;162:1267–76.
Nides M, Shanga GM, Bishop A, Becker WD. Nicotine lozenges in the relief of behaviorally provoked craving. Am J Health Behav. 2018;42:69–79.
Acknowledgements
We would like to acknowledge and thank the subjects for their participation in these studies.
Funding
Sponsorship for this study and Rapid Service Fee were funded by GlaxoSmithKline.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Pamela M Lai contributed to the concept and design of the study and drafting of the manuscript; Mako Araga contributed to the concept and design of the study, the statistical analysis, and the drafting of the manuscript; Ana Hamilton contributed to the concept and design of the study and the drafting of the manuscript; Marianna Armogida contributed to the concept and design of the study and the drafting of the manuscript.
Medical Writing and/or Editorial Assistance
Editorial assistance in the preparation of this article was provided by Andrea S. Blevins Primeau, PhD, MBA, of Medica Communications Inc. Support for this assistance was funded by GlaxoSmithKline.
Disclosures
Pamela M Lai, Mako Araga, Ana Hamilton, and Marianna Armogida are employees of GlaxoSmithKline.
Compliance with Ethics Guidelines
The studies were reviewed and approved by an independent institutional review board: Advarra IRB, 6940 Columbia Gateway Drive, Suite 110, Columbia, Maryland, 21046, USA (IRB# 00000971; IORG # 0000635). The studies were conducted in full compliance of all relevant laws and regulations in the USA and with the requirements specified in the Declaration of Helsinki, and in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. All subjects provided written informed consent prior to any study-specific procedures. Subjects could withdraw from either study at any time for any reason.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, P.M., Araga, M., Hamilton, A. et al. Pharmacokinetic Characterization of a Prototype Mini Nicotine Lozenge. Adv Ther 38, 3997–4012 (2021). https://doi.org/10.1007/s12325-021-01798-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01798-4