Abstract
Rationale
Current nicotine replacement products provide a much slower onset of nicotine delivery than cigarettes, and hence are only marginally effective at supplanting cigarette smoking. Therefore, more effective forms of nicotine replacement are needed.
Objectives
This initial investigation characterized the pharmacokinetic (PK) and subjective effects of a novel sublingual (SL) nicotine tablet designed to deliver nicotine more rapidly to the bloodstream of smokers.
Methods
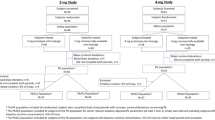
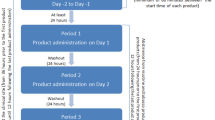
Study 1 (N = 6) characterized the pharmacokinetics of a 2 mg nicotine SL tablet in comparison to an FDA-approved, marketed 2 mg nicotine lozenge. Study 2 (N = 24) assessed subjective responses of smokers to a single use of a 1 mg and 2 mg SL tablet.
Results
Study 1 found that the time to maximum blood nicotine concentrations was significantly shorter for the SL tablet (14 min) than for the lozenge (82 min), and the initial rate of nicotine absorption was higher (0.4 ng/mL*min vs. 0.0 ng/mL*min), supporting the hypothesis that the SL tablet delivered nicotine more rapidly. Study 2 found that participants reported immediate relief of nicotine withdrawal symptoms after tablet administration, and craving reduction after the 2 mg tablet approached the degree reported for their usual brands of cigarettes (4.2 vs. 4.6 on a 7-point scale). Other subjective responses showed the tablet to be an appealing alternative to smoking.
Conclusions
The novel SL tablet studied shows promise as a nicotine substitution strategy for tobacco harm reduction and smoking cessation treatment. Additional studies are warranted to further investigate the potential of this new approach.



Similar content being viewed by others
References
Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS (2018) Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Public Health 39(1):193–213. https://doi.org/10.1146/annurev-publhealth-040617-013849
Adriaens K, Gucht DV, Baeyens F (2018) IQOSTM vs. e-cigarette vs. tobacco cigarette: a direct comparison of short-term effects after overnight-abstinence. Int J Environ Res Public Health 15(12):2902. https://doi.org/10.3390/ijerph15122902
Amodei N, Lamb RJ (2008) Over-the-counter nicotine replacement therapy: can its impact on smoking cessation be enhanced? Psychol Addict Behav 22(4):472–485. https://doi.org/10.1037/0893-164X.22.4.472
Benowitz NL, Hukkanen J, Jacob P (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. In: Henningfield JE, London ED, Pogun S (eds) Nicotine psychopharmacology, vol 192. Springer Berlin, Heidelberg, pp 29–60. https://doi.org/10.1007/978-3-540-69248-5_2
Bergeria CL, Heil SH, Davis DR, Streck JM, Sigmon SC, Bunn JY, Tidey JW, Arger CA, Reed DD, Gallagher T, Hughes JR, Gaalema DE, Stitzer ML, Higgins ST (2019) Evaluating the utility of the modified cigarette evaluation questionnaire and cigarette purchase task for predicting acute relative reinforcing efficacy of cigarettes varying in nicotine content. Drug Alcohol Depend 197:56–64. https://doi.org/10.1016/j.drugalcdep.2019.01.004. Epub 2019 Feb 13
Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A (1999) Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow up. BMJ (Clin Res Ed) 318(7179):285–288. https://doi.org/10.1136/bmj.318.7179.285
Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG (2007) Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav 32(5):912–923. https://doi.org/10.1016/j.addbeh.2006.06.028
Choi J, Dresler C, Norton M, Strahs K (2003) Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob Res 5(5):635–644. https://doi.org/10.1080/1462220031000158690
Chow S-C, Liu J (2009) Design and analysis of bioavailability and bioequivalence studies, 3rd edn. CRC Press
Dautzenberg B, Nides M, Kienzler J-L, Callens A (2007) Pharmacokinetics, safety and efficacy from randomized controlled trials of 1 and 2 mg nicotine bitartrate lozenges (Nicotinell®). BMC Clin Pharmacol 7(1):11. https://doi.org/10.1186/1472-6904-7-11
de Wit H, Dudish, S, Ambre, J (1993) Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology 112:324–330. https://doi.org/10.1007/BF02244928
Ebajemito JK, McEwan M, Gale N, Camacho OM, Hardie G, Proctor CJ (2020) A randomised controlled single-centre open-label pharmacokinetic study to examine various approaches of nicotine delivery using electronic cigarettes. Sci Rep 10(1):19980. https://doi.org/10.1038/s41598-020-76610-4
Etter J-F (2006) Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 15(4):280–285. https://doi.org/10.1136/tc.2005.015487
Feyerabend C, Ings R, Russel M (1985) Nicotine pharmacokinetics and its application to intake from smoking. Br J Clin Pharmacol 19(2):239–247. https://doi.org/10.1111/j.1365-2125.1985.tb02637.x
Gottlieb S, Zeller M (2017) A nicotine-focused framework for public health. N Engl J Med 377(12):1111–1114. https://doi.org/10.1056/NEJMp1707409
Grady SR, Marks MJ, Collins AC (2008) Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. J Neurochem 62(4):1390–1398. https://doi.org/10.1046/j.1471-4159.1994.62041390.x
Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T (2018) Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 2019(1). https://doi.org/10.1002/14651858.CD000146.pub5
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 86(9):1119–27. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x
Hua S (2019) Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Front Pharmacol 10:1328. https://doi.org/10.3389/fphar.2019.01328
Hughes JR (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43(3):289. https://doi.org/10.1001/archpsyc.1986.01800030107013
Hughes J, Hatsukami DK (1998) Errors in using tobacco withdrawal scale. Tob Control 7(1):92–93. https://doi.org/10.1136/tc.7.1.92a
Jackson SE, McGowan JA, Ubhi HK, Proudfoot H, Shahab L, Brown J, West R (2019) Modelling continuous abstinence rates over time from clinical trials of pharmacological interventions for smoking cessation. Addiction 114(5):787–797. https://doi.org/10.1111/add.14549
Koranda JL, Cone JJ, McGehee DS, Roitman MF, Beeler JA, Zhuang X (2014) Nicotinic receptors regulate the dynamic range of dopamine release in vivo. J Neurophysiol 111(1):103–111. https://doi.org/10.1152/jn.00269.2013
Kotlyar M, Lindgren BR, Vuchetich JP, Le C, Mills AM, Amiot E, Hatsukami DK (2017) Timing of nicotine lozenge administration to minimize trigger induced craving and withdrawal symptoms. Addict Behav 71:18–24. https://doi.org/10.1016/j.addbeh.2017.02.018
McCarty JA (2015) Transmucosal drug delivery system (Patent No. US 8,992,974)
McNeill A, Foulds J, Bates C (2001) Regulation of nicotine replacement therapies (NRT): a critique of current practice. Addiction (Abingdon, England) 96(12):1757–1768. https://doi.org/10.1080/09652140120089508
Mersha AG, Eftekhari P, Bovill M, Tollosa DN, Gould GS (2021) Evaluating level of adherence to nicotine replacement therapy and its impact on smoking cessation: a systematic review and meta-analysis. Arch Public Health 79(1):26. https://doi.org/10.1186/s13690-021-00550-2
Molander L, Lunell E (2001) Pharmacokinetic investigation of a nicotine sublingual tablet. Eur J Clin Pharmacol 56(11):813–819. https://doi.org/10.1007/s002280000223
Morgan JC, Cappella JN (2021) Harm perceptions and beliefs about potential modified risk tobacco products. Int J Environ Res Public Health 18(2):576. https://doi.org/10.3390/ijerph18020576
Murray RP, Connett JE, Zapawa LM (2009) Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res 11(9):1076–1082. https://doi.org/10.1093/ntr/ntp104
Olsson Gisleskog PO, Perez Ruixo JJ, Westin Å, Hansson AC, Soons PA (2021) Nicotine population pharmacokinetics in healthy smokers after intravenous, oral, buccal and transdermal administration. Clin Pharmacokinet 60(4):541–561. https://doi.org/10.1007/s40262-020-00960-5
Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84(4):329–342. https://doi.org/10.1016/j.pneurobio.2007.12.005
Rose JE, Behm FM, Westman EC, Bates JE, Salley A (2003) Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav 76(2):243–250. https://doi.org/10.1016/j.pbb.2003.07.002
Rosen LJ, Galili T, Kott J, Rees V (2021) Beyond “safe and effective”: the urgent need for high-impact smoking cessation medications. Prev Med 150:106567. https://doi.org/10.1016/j.ypmed.2021.106567
Shahab L, Dobbie F, Hiscock R, McNeill A, Bauld L (2016) Prevalence and impact of long-term use of nicotine replacement therapy in UK Stop-Smoking Services: findings from the ELONS Study. Nicotine Tobacco Res ntw58. https://doi.org/10.1093/ntr/ntw258
Shiffman S, Hughes JR, Di Marino ME, Sweeney CT (2003) Patterns of over-the-counter nicotine gum use: persistent use and concurrent smoking: misuse of nicotine gum. Addiction 98(12):1747–1753. https://doi.org/10.1111/j.1360-0443.2003.00575.x
Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG (2008) Use of smoking-cessation treatments in the United States. Am J Prev Med 34(2):102–111. https://doi.org/10.1016/j.amepre.2007.09.033
Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE (2017) Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology 234(17):2643–2655. https://doi.org/10.1007/s00213-017-4665-y
Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S (2007) Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav 21(2):216–225. https://doi.org/10.1037/0893-164X.21.2.216
Ward KD, Garvey AJ, Bliss RE (1992) Evidence of transient heart rate change after smoking cessation. Psychopharmacology 106(3):337–340. https://doi.org/10.1007/BF02245414
West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A (2000) A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology 149:198–202
Westman EC, Levin ED, Rose JE (1992) Smoking while wearing the nicotine patch-is smoking satisfying or harmful. Clin Res 40(4):A871–A871
Funding
Study 1 was funded by the National Institute of Health via a National Institute on Drug Abuse STTR Grant 1R41DA033710-01A1, and Study 2 was funded by Nicotine BRST LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JER and FMB disclose research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, Embera Neurotherapeutics, Inc., Otsuka Pharmaceutical, JUUL Labs, consulting with Revive pharmaceuticals, and consulting and patent purchase agreement with Philip Morris International.
JM is Nicotine BRST’s Chief Scientific Officer at which he holds an equity interest.
FV has consulted with Revive Therapeutics.
TLB, DRB, and PNW disclose research support from Foundation for a Smoke-Free World, Philip Morris International, Altria, Embera Neurotherapeutics, Inc., Otsuka Pharmaceutical, and JUUL Labs.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rose, J.E., Behm, F.M., Botts, T.L. et al. Novel rapid-acting sublingual nicotine tablet as a cigarette substitution strategy. Psychopharmacology 239, 2853–2862 (2022). https://doi.org/10.1007/s00213-022-06171-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06171-z