Abstract
The presence of rare comorbidities in patients with cardiovascular disease (CVD) presents a diagnostic challenge to cardiologists. In evaluating these patients, cardiologists are faced with a unique opportunity to shorten diagnosis times and direct patients towards correct treatment pathways. Idiopathic pulmonary fibrosis (IPF), a type of interstitial lung disease (ILD), is an example of a rare disease where patients frequently demonstrate comorbid CVD. Both CVD and IPF most commonly affect a similar patient demographic: men over the age of 60 years with a history of smoking. Moreover, IPF and heart failure (HF) share a number of symptoms. As a result, patients with IPF can be misdiagnosed with HF and vice versa. This article aims to increase awareness of IPF among cardiologists, providing an overview for cardiologists on the differential diagnosis of IPF from HF, and describing the signs and symptoms that would warrant referral to a pulmonologist with expertise in ILD. Once patients with IPF have received a diagnosis, cardiologists can have an important role in managing patients who are candidates for a lung transplant or those who develop pulmonary hypertension (PH). Group 3 PH is one of the most common cardiovascular complications diagnosed in patients with IPF, its prevalence varying between reports but most often cited as between 30% and 50%. This review summarizes the current knowledge on Group 3 PH in IPF, discusses data from clinical trials assessing treatments for Group 1 PH in patients with IPF, and highlights that treatment guidelines recommend against these therapies in IPF. Finally, this article provides the cardiologist with an overview on the use of the two approved treatments for IPF, the antifibrotics pirfenidone and nintedanib, in patients with IPF and CVD comorbidities. Conversely, the impact of treatments for CVD comorbidities on patients with IPF is also discussed.
Funding: F. Hoffmann-La Roche, Ltd.
Plain Language Summary: Plain language summary available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain Language Summary
Many patients with heart disease also have other medical conditions. This makes it harder for doctors specialized in treating heart disease (cardiologists) to treat these patients.
Idiopathic pulmonary fibrosis (IPF) is a rare lung disease. Patients with IPF often also have heart disease.
Many patients with IPF have to wait a long time to be diagnosed and sometimes they are given an incorrect diagnosis, for example, heart disease. This is because IPF and some types of heart disease have similar symptoms and both diseases most commonly affect men aged over 60 years who smoke or used to smoke. Raising awareness of IPF among cardiologists could help to reduce the number of patients with IPF receiving an incorrect diagnosis and could reduce the time it takes to receive a diagnosis.
Some patients have both IPF and heart disease. This is important because different heart diseases can affect the choice of medicines to prescribe for IPF. For example, many patients with heart disease also have kidney problems and other patients might have bleeding problems. Both of these factors might influence medication choice.
This article aims to raise awareness of IPF among cardiologists. It describes the signs and symptoms of IPF, and provides instructions to help cardiologists decide if a patient might have IPF. This article also provides information to help cardiologists decide what medicines to prescribe to patients who have both IPF and heart disease, and highlights the importance of doctors working together when they are treating the same patient.
Introduction
The presence of rare comorbid diseases in patients with cardiovascular disease (CVD) can present a diagnostic challenge to cardiologists. Idiopathic pulmonary fibrosis (IPF) is a type of interstitial lung disease (ILD; Fig. 1) and is an example of a rare disease where patients frequently demonstrate comorbid CVD [1,2,3,4,5,6]. Like CVD, IPF affects more men than women and is more frequent in current or ex-smokers, and the majority of diagnoses occur in patients over the age of 60 years [7,8,9,10,11]. In addition, IPF presents with a number of non-specific symptoms, for example, dyspnea, cough, and reduced exercise capacity, many of which can be mistaken for symptoms of heart failure (HF) [12, 13]. Pulmonologists are the specialists responsible for the definitive diagnosis and treatment of IPF. However, cardiologists may be in a privileged position to identify patients with a potential diagnosis of IPF earlier and refer them to pulmonologists, and thereby reduce the number of misdiagnoses, shorten diagnosis times, and direct patients towards correct treatment pathways [12, 14,15,16].

Reprinted with permission of the American Thoracic Society. Copyright © [2018] American Thoracic Society [121, 123].
Classification of ILDs [121,122,123]. EP eosinophilic pneumonia, LAM lymphangioleiomyomatosis, LCH Langerhans cell histiocytosis. aClinical, radiological, pathological. This figure is based on previously published information. Permission for re-use has been granted for: Ryerson and Collard [122], https://journals.lww.com/co-pulmonarymedicine/Abstract/2013/09000/Update_on_the_diagnosis_and_classification_of_ILD.8.aspx.
IPF is a debilitating, irreversible, and fatal ILD characterized by the formation of scar tissue and architectural distortion in the lungs, with a progressive decline in lung function and a median survival following diagnosis of between 2 and 5 years [6, 11, 17]. The cause of IPF is unknown and its clinical course is variable, with no way to accurately predict prognosis [1, 17]. The incidence of IPF in Europe and North America has been estimated to be between 3 and 9 cases per 100,000 of the population per year, and evidence suggests that the prevalence is increasing over time [18,19,20]. Currently, there are no treatments capable of reversing fibrotic lung damage in patients with IPF. However, there are two approved treatments for patients with IPF, the antifibrotics pirfenidone and nintedanib [21,22,23,24]. Both have been shown to significantly reduce lung function decline versus placebo over 52 weeks [25, 26]. In selected patients, lung transplantation (LTx) can help to prolong survival and improve functional status [11, 27, 28]. The early and correct diagnosis of IPF is instrumental to patients accessing treatments with the potential to slow disease progression and prolong survival.
Increased awareness of IPF is required across a range of clinical specialties to help the identification, diagnosis, and treatment [29]. This review aims to raise awareness of IPF among cardiologists by exploring a number of topics that are relevant to the cardiology community, including: differential diagnosis between IPF and HF; the prevalence of cardiovascular complications in patients with IPF, with a focus on pulmonary hypertension (PH); and the management of patients with comorbid IPF and CVD. This article does not contain any studies with human participants or animals performed by any of the authors.
Diagnosis of IPF
Diagnostic Delays in Patients with IPF
The diagnosis of IPF is often a protracted and challenging process, with many patients enduring extended delays before receiving a diagnosis [14, 30,31,32]. For example, some patients with IPF may be symptomatic up to 5 years before diagnosis, exhibiting breathlessness and/or cough [15]. In a review of referral letters of patients with IPF identified from the Finnish IPF registry, the mean (range) time between symptom onset and referral to a pulmonologist was 1.5 (0.8–2.3) years [32]. Similarly, in a prospective cohort study including 129 patients with IPF in the US, the median (interquartile range) reported delay between symptom onset and evaluation at a tertiary center was 2.2 (1.0–3.8) years [31]. The 2018 international (ATS/ERS/JRS/ALAT) guidelines describe the complexities of the diagnosis of IPF that, by its nature, is one of exclusion (Fig. 2) [1].
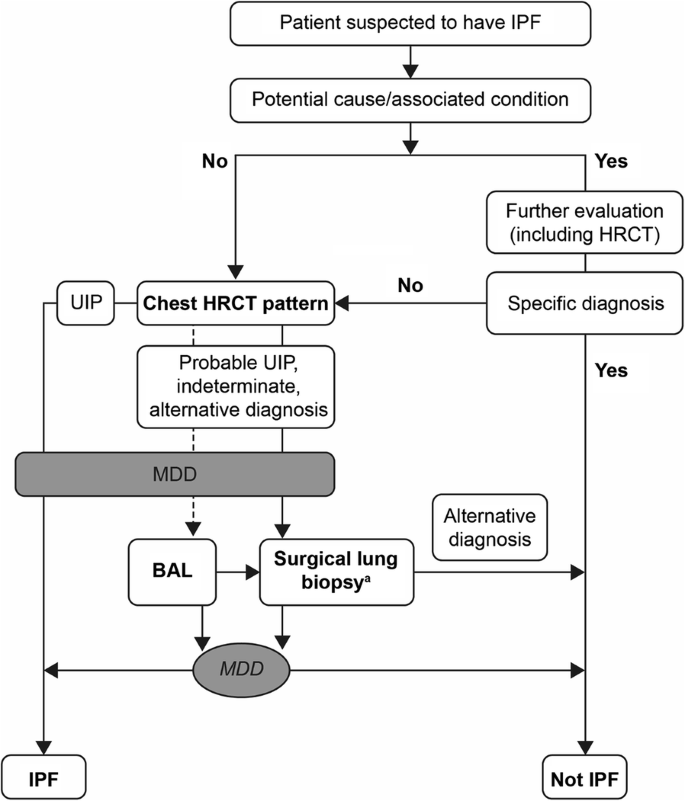
Reprinted with permission of the American Thoracic Society. Copyright © [2018] American Thoracic Society [1]. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society
Diagnostic algorithm for IPF from 2018 ATS/ERS/JRS/ALAT guidelines [1]. ALAT Latin American Thoracic Society, ATS American Thoracic Society, BAL bronchoalveolar lavage, ERS European Respiratory Society, HRCT high-resolution computed tomography, IPF idiopathic pulmonary fibrosis, JRS Japanese Respiratory Society, MDD multidisciplinary discussion, UIP usual interstitial pneumonia. aSurgical lung biopsy is not indicated in patients at high risk for intra-, peri-, or post-operative complications.
In particular, diagnostic delays may be introduced if patients are misdiagnosed or prescribed multiple treatments for other conditions before they are finally referred to an ILD specialist. For example, in two surveys of patients with pulmonary fibrosis, over 50% of patients reported an initial misdiagnosis. Although other respiratory conditions, such as bronchitis and asthma, were more likely to be misdiagnosed, heart disease was also a misdiagnosis in some patients [14, 16]. Delays in diagnosis have been associated with negative outcomes in patients with IPF, with longer delays associated with an increased risk of death independent of lung function [31]. Similarly, real-world results from the European EMPIRE registry have demonstrated a reduced median survival in patients who waited over 12 months for a diagnosis of IPF versus those who waited less than 12 months [33].
Differential Diagnosis of IPF and HF
IPF presents with non-specific symptoms such as dyspnea, which can be present in a variety of other conditions, including HF, asthma, chronic obstructive pulmonary disease, pulmonary thromboembolism, or pneumonia [11, 12]. In particular, IPF and HF can be particularly challenging to distinguish from each other [34], as both conditions commonly present with dyspnea and reduced exercise tolerance (Table 1) [6, 12, 35]. Furthermore, there is considerable overlap between the populations most affected by IPF and CVD, with both conditions being more common in men over the age of 60 years with a history of smoking [7,8,9,10,11].
A detailed medical history and physical examination can provide a number of useful observations, which may help to guide further investigations and distinguish IPF from HF (Table 2). Dyspnea accompanied by bilateral edema, orthopnea, increased jugular venous pressure, a displaced apical beat, and bilateral posterior inspiratory crepitations/smooth crackles at the lung bases in a patient with a history of coronary artery disease (CAD), arterial hypertension, or diuretic use would obviously warrant further investigation of a diagnosis of HF [12, 35]. However, dyspnea accompanied by fine ‘Velcro®’ crackles on auscultation at the posterior lung bases, or evidence of clubbed fingers, which are present in 25–50% of patients with IPF, would instead indicate that the patient should be referred to an ILD specialist for further investigation [7, 12, 13]. Velcro® crackles, considered characteristic of IPF, have been compared with the sound of gently separating Velcro®, and are thought to be the first abnormality on physical examination of patients with IPF [36]. Velcro® crackles in IPF are bibasal, mostly present for the entire inspiratory time (pan-inspiratory), fine in quality, and persist after deep breaths and coughing. As IPF progresses they may be auscultated higher in the bases and heard throughout the lower lobes [36]. Although Velcro® crackles are not specific for IPF, their presence accompanied by dyspnea, gas-exchange abnormalities, and lung infiltrates should prompt referral to an ILD specialist [36]. Similarly, referral should be considered if crackles are not accompanied by ancillary features associated with HF (for example, peripheral edema, elevated central venous pressure) or bilateral pneumonia (for example, fever, colored phlegm).
In patients in whom a detailed medical history and physical examination does not lead to an obvious diagnosis, there are a number of additional tests that can exclude or confirm diagnoses of IPF or HF (Table 2). Plasma levels of natriuretic peptides, such as brain natriuretic peptide (BNP) or N-terminal prohormone BNP (NT-proBNP), are extremely useful for the diagnosis of HF [12, 35, 37]. HF can typically be excluded in patients with normal levels of BNP or NT-proBNP (BNP ≤ 35 pg/ml and/or NT-proBNP ≤ 125 pg/ml) [35]; however, it should be noted that elevated levels do not necessarily rule out a diagnosis of IPF [38, 39]. When elevated levels of BNP or NT-proBNP are confirmed, echocardiography is mandatory to confirm structural or functional cardiac abnormalities [35].
To investigate a potential diagnosis of IPF, lung function and oxygen saturation should be measured (Table 2) [11]. Patients with IPF typically demonstrate impaired lung function on spirometry, with reductions in forced vital capacity (FVC) and carbon monoxide diffusing capacity (DLco) being the most common findings [11, 40, 41], although some patients with HF may also show lung function abnormalities. Mild left HF is a recognized cause of increased DLco, due to increased pulmonary capillary blood flow; more significant HF is associated with restriction on pulmonary function tests and a low DLco, and patients may have mild obstruction on spirometry arising from small airway edema [41, 42]. Hypoxemia is highly suggestive of IPF as it is extremely rare in patients with isolated HF, and can be detected through the measurement of arterial blood gases [7]. In the cardiology outpatient setting, where measurement of arterial blood gases may not be feasible, pulse oximetry is usually a readily available method to identify gas-exchange abnormalities [7].
Ultimately, imaging of the chest is required to confirm a diagnosis of IPF (Table 2). If a chest X-ray is available, decreased lung volumes and subpleural reticular opacities that increase from the apex to the base of the lung are likely to be observed in patients with IPF [43]. However, a normal chest X-ray does not rule out IPF and, therefore, in the correct clinical setting, diagnosis of IPF should be confirmed with a high-resolution computed tomography (HRCT) image of the chest, with a pattern of typical or probable usual interstitial pattern (UIP) considered diagnostic (Table 3) [44]. UIP is defined as the presence of reticular opacities and clustered cystic airspaces, referred to as honeycombing, which are typically found in the basal and peripheral regions of the lungs [11]. However, it should be noted that the presence of a typical UIP pattern on HRCT is not specific for a diagnosis of IPF because it can also be present in other ILDs, for example, chronic hypersensitivity pneumonitis and connective tissue disease-associated ILD [45]. It is therefore imperative that a pulmonologist conducts a comprehensive clinical evaluation to identify any potential known causes of ILD. As confirming a diagnosis of IPF can be cumbersome, experts recommend the involvement of an ILD multidisciplinary team to establish an IPF diagnosis, typically involving a pulmonologist, a radiologist, and a pathologist (all experts in ILD within their specialty), among other members [1].
Comorbidities and Complications in Patients with IPF
Cardiovascular Comorbidities in Patients with IPF
It is important to note that, in many patients, CVD is diagnosed before IPF [46]. Comprehensive cardiovascular evaluation represents an opportunity for cardiologists to identify undiagnosed IPF in patients with CVD and refer them to a pulmonologist with expertise in ILD.
Patients with IPF demonstrate a high burden of CVD, with a number of studies reporting an increased risk of CVD in patients with IPF versus those without [3,4,5, 47]. Although respiratory failure is the most frequent cause of death in patients with IPF, CVD is still responsible for up to 10% of deaths [39, 48, 49]. The presence of cardiovascular comorbidities in patients with IPF and the effect on mortality have been investigated in several studies. For example, in a retrospective study of patients with pulmonary fibrosis treated at a single hospital in Finland, CVD remained associated with increased mortality in multivariate analyses adjusted for age, gender, smoking status, and percent predicted DLco [46]. Similarly, in a study including data from 272 patients with IPF from an ILD tertiary referral center, atherosclerosis and ‘other’ cardiac diseases were associated with increased mortality [50].
PH in Patients with IPF
PH is currently classified into five categories, which are differentiated by multiple factors including hemodynamic characteristics and pathological findings [51]. Group 3 PH is associated with lung disease and is a common cardiovascular complication diagnosed in patients with IPF, with prevalence varying between 3% and 86% but most often found to be between 30% and 50% [39, 52,53,54,55,56,57]. The presence of PH in patients with IPF is associated with a number of negative outcomes. For example, in a retrospective cohort study including data obtained from a US healthcare database on 6013 LTx candidates with IPF who were followed-up until death, LTx, or any other censoring event, ‘mild’ and ‘severe’ PH were significantly associated with mortality [58].
The pathophysiologic mechanisms accounting for PH in patients with IPF are complex [39]. Hypoxemic vasoconstriction and destruction of the pulmonary vascular bed by fibrosis, as well as aberrant angiogenesis and endothelial dysfunction, are likely to influence the development of PH [39, 59, 60]. However, the biological processes underlying progressive fibrogenesis may also be contributing factors as profibrogenic cytokines are also vasoactive [39, 59,60,61,62]. In addition, other common comorbidities in patients with IPF, such as emphysema, obstructive sleep apnea (OSA), thromboembolic disease, and HF are also likely to contribute to the development of PH [39]. In particular, the fibrotic lung tissue from patients with IPF is reported to increase levels of coagulation factors and their downstream activators [63, 64]. Venous thromboembolism has been used as a proxy for such a ‘procoagulant state’ and has been linked to interstitial idiopathic pneumonia (of which IPF is part; Fig. 1), especially amongst those never treated with anticoagulants [65].
The symptoms of PH are non-specific and overlap with those of IPF, including dyspnea, exercise intolerance, and fatigue [39, 66]. This means that many patients with IPF are not evaluated for PH [39, 66]. The presence of PH should, however, be considered in every patient with IPF, especially when dyspnea or oxygen desaturation is disproportionate to the physiologic impairment demonstrated on pulmonary function testing or the findings on HRCT imaging [39, 53]. Other indicators of PH may include percent predicted DLco < 30%, unexpected reductions in 6 min walk distance (6MWD) often with oxygen desaturation to below 85%, and impaired heart-rate recovery after exertion [39, 53]. Although right-heart catheterization is considered the gold standard of diagnosis for Group 1 PH (pulmonary arterial hypertension), it is not systematically recommended in patients with IPF who exhibit the signs and symptoms of PH unless the patient is being considered for LTx, or if it is clinically indicated [56, 66]. When PH is suspected, quantification of NT-proBNP levels in patients with IPF can provide confirmation of whether further investigation is necessary [67]. A diagnosis of PH becomes very unlikely in patients with NT-proBNP < 95 ng/l [67]. In patients with NT-proBNP ≥ 95 ng/l, a transthoracic echocardiogram can be used to look for signs of elevated right ventricular systolic pressure, such as dilation of the right atrium and/or ventricle, and right ventricular dysfunction [39]. The echocardiographic probability of PH can be defined as low, intermediate, or high based on a combination of peak tricuspid regurgitation velocity and the presence or absence of signs of PH on echocardiogram [56]. HRCT images of the chest may also assist with the identification of PH when showing a main pulmonary artery diameter > 29 mm or a pulmonary artery diameter greater than that of the aorta [66].
There are a number of therapies available for Group 1 PH, including calcium channel blockers, endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostacyclin analogs [56]. However, international treatment guidelines for IPF and PH do not recommend treating Group 3 PH in patients with IPF with the therapies available for Group 1 PH because of a lack of clinical evidence supporting the efficacy and safety of these treatments and the potential risk of impairing gas exchange through the inhibition of hypoxic vasoconstriction [56, 68]. Nevertheless, the guidelines do recommend that patients with IPF and PH and who are hypoxemic should receive long-term oxygen therapy [11, 56].
A number of randomized controlled trials have investigated therapies for Group 1 PH in patients with IPF and ILD with mixed and sometimes deleterious results (a comprehensive list is found in Table 4). For example, the RISE-IIP study of riociguat in patients with idiopathic interstitial pneumonia and PH was terminated early due to an increased risk of death or serious adverse events (AEs) in the active treatment group [39, 69]. The STEP-IPF trial of sildenafil in patients with advanced IPF, defined as percent predicted DLco < 35%, was completed but did not show a significant treatment benefit for the primary endpoint of the proportion of patients with an increase in 6MWD ≥ 20% at week 12 [70]. However, significant treatment benefits were observed for secondary endpoints versus placebo, including changes in arterial oxygenation, percent predicted DLco, dyspnea, and quality of life (QoL) after 12 weeks of treatment [70]. The use of sildenafil in combination with antifibrotics has recently attracted attention based on two randomized placebo-controlled clinical trials of patients with IPF [71, 72]. NCT02951429 is enrolling patients with IPF with more advanced disease (percent predicted DLco ≤ 40%) at risk of Group 3 PH, and will investigate the efficacy, safety, and tolerability of sildenafil added to pirfenidone over 52 weeks [71, 73]. The primary outcome is the percentage of patients with disease progression, defined as the occurrence of ≥ 15% decline in 6MWD, respiratory-related non-elective hospitalization, or all-cause mortality. Another trial, INSTAGE (NCT02802345), enrolled patients with IPF and advanced lung-function impairment (percent predicted DLco ≤ 35%), and investigated the efficacy and safety of sildenafil added to nintedanib over 24 weeks [72]. The primary outcome was the change from baseline at week 12 in St George’s Respiratory Questionnaire (SGRQ) total score. These results were recently reported and showed that the difference in change from baseline in the SGRQ total score between the nintedanib and sildenafil treatment arm and the nintedanib alone arm was not significant at weeks 12 or 24 [74]. A large number of exploratory outcomes showed no benefit of adding sildenafil to treatment with nintedanib, with the exception that patients treated with nintedanib plus sildenafil had a lower risk of reaching a composite endpoint of absolute decline in percent predicted FVC of ≥ 5% or death than those treated with nintedanib alone [74]. The absence of an increase in BNP level in the patients who received nintedanib plus sildenafil in the trial may indicate a reduction in right ventricular stress [74].
Other Comorbidities in Patients with IPF
In addition to CVD and PH, other comorbidities, such as gastroesophageal reflux disease (GERD) and OSA, are frequently associated with IPF [75]. The precise prevalence of GERD amongst patients with IPF is difficult to ascertain because of differences in diagnostic procedures, but it may affect over 80% of individuals [76, 77]. It has been hypothesized that GERD may contribute to the progression of IPF in some patients and studies exploring the effect of anti-acid therapy have been performed. In an analysis of the placebo arms of three IPF Clinical Research Network randomized clinical trials, those patients taking anti-acid medication at baseline had a slower decline in percent predicted FVC over 30 weeks [78]. A post hoc analysis of a separate clinical trial data set did not replicate this result and a more recent Phase II clinical trial of omeprazole in patients with IPF has yet to report (NCT02085018) [79, 80].
OSA is a frequent comorbid condition in patients with IPF, with a reported prevalence between 58% and 88% [53]. Despite this high reported prevalence, surprisingly few patients are evaluated for OSA [53]. If left untreated, OSA can result in nocturnal hypoxemia, the presence of which was recently shown to predict worsened survival in patients with IPF [81]. Moderate-to-severe OSA is generally treated with continuous positive airway pressure and this treatment has been shown to improve QoL measures in patients with IPF [53].
Management of Patients with IPF: Considerations for Cardiologists
Antifibrotics
Two therapies are currently available for the treatment of patients with IPF, the antifibrotics pirfenidone and nintedanib [21,22,23,24]. Pirfenidone is an antifibrotic, anti-inflammatory, and anti-oxidant compound [82]. Its direct mechanism of action in IPF is not fully established [82]. Nintedanib is a tyrosine kinase inhibitor, which mainly inhibits receptors for platelet-derived growth factor, fibroblast growth factor, and vascular endothelial growth factor (VEGF) [83]. Neither treatment can reverse the fibrotic damage associated with IPF. However, both pirfenidone and nintedanib have been shown to reduce lung-function decline versus placebo in pivotal Phase III clinical trials [25, 26]. A pooled analysis of the pirfenidone pivotal Phase III trials, ASCEND and CAPACITY, demonstrated that, at 1 year and compared with placebo, pirfenidone reduced the proportion of patients with a decline in percent predicted FVC or death by 44% [26]. In the nintedanib pivotal Phase III trials, nintedanib reduced the annual rate of change in FVC versus placebo by 125 ml in INPULSIS-1 and by 94 ml in INPULSIS-2 [25].
Considering survival, the pirfenidone pivotal Phase III trials were not individually powered to show a difference, but a pooled analysis and meta-analyses concluded that pirfenidone significantly reduced mortality versus placebo over 120 weeks [84]. A meta-analysis of the nintedanib Phase II TOMORROW and Phase III INPULSIS trials found trends for reduced mortality versus placebo over 52 weeks [85]. It remains unknown to what extent increased use of antifibrotics may influence long-term survival in IPF.
The safety and tolerability of pirfenidone and nintedanib have been extensively characterized, and the most common AEs with both drugs are gastrointestinal, with the potential for rashes with pirfenidone [85, 86]. No clinical trial has, as yet, specifically explored the use of antifibrotics in patients with IPF and cardiovascular comorbidity. However, the cardiovascular safety and tolerability profiles of pirfenidone and nintedanib are evidenced by findings from the pivotal Phase III trials.
There is a minor concern that the effect of nintedanib on VEGF may lead to a small increased risk of non-serious bleeding events and the US and European product labels specify that patients at known risk for bleeding should only receive nintedanib if the anticipated benefit outweighs the risk [21, 24, 87]. Cardiologists may wish to consider the potential for bleeding events in their patients who are taking nintedanib for IPF and who require anticoagulation or antiplatelet therapy for comorbid conditions [21]. In the Phase III pivotal INPULSIS trials of nintedanib, where patients at known risk for bleeding were excluded, serious bleeding events had a low incidence and occurred at a similar frequency in both treatment arms (nintedanib 1.3% and placebo 1.4%) [21]. Of note, the most frequent bleeding event was non-serious epistaxis, a finding that is supported by post-marketing surveillance data [88]. Caution is urged when prescribing nintedanib in patients with IPF and a history of abdominal surgery, peptic ulceration, or diverticular disease, and patients who are prescribed corticosteroids or non-steroidal anti-inflammatory medications [21]. Regular monitoring of blood pressure in patients treated with nintedanib is also recommended in Europe, presumably because nintedanib inhibits the VEGF pathway and oncology studies have shown that anti-VEGF therapy frequently leads to hypertension in patients with renal cancer [21, 89, 90]. When prescribing pirfenidone in patients with IPF, there are no special warnings or precautions surrounding bleeding events that require consideration [22]. In a retrospective blinded review of data from the pivotal Phase III ASCEND and CAPACITY clinical trials, presented as a conference abstract (not yet subjected to peer review in the form of an original research article), the pooled incidence of bleeding events was 3.7% and 4.3% in the pirfenidone and placebo treatment arms, respectively [91].
The more general cardiovascular safety and tolerability of pirfenidone and nintedanib were also assessed in the Phase III clinical trials. In the same retrospective blinded review of data from ASCEND and CAPACITY, again presented as a conference abstract (not yet subjected to peer review in the form of an original research article), the pooled incidence of major adverse cardiovascular events (cardiac arrest, myocardial infarction, stroke, and unstable angina) was 1.4% and 2.1% in the pirfenidone and placebo arms, respectively [92]. In the INPULSIS trials, cardiac AEs were reported by 10.0% and 10.6% of patients treated with nintedanib and placebo, respectively, and serious cardiac AEs were reported by 5.0% and 5.4% of patients, respectively [25]. Real-world evidence for both pirfenidone and nintedanib has suggested that the safety and tolerability of these drugs in clinical practice is similar to the Phase III trials [93,94,95,96,97,98,99]. Furthermore, the ongoing clinical trials should provide more extensive information on the effect of these antifibrotic drugs in combination with treatments for PH [100].
A further consideration for cardiologists and pulmonologists making prescribing decisions in patients with cormorbid IPF and heart disease is renal disease. Findings from clinical studies demonstrate that patients with heart failure frequently have renal impairment [101, 102]. The use of antifibrotics in patients with IPF and renal impairment has not been specifically studied, and the Phase III clinical trials present little or no information on renal AEs [25, 103, 104]. According to the US product label, pirfenidone should be used with caution in patients with any renal impairment, and its use is not recommended in patients with end-stage renal disease requiring dialysis [23]. According to the European product label, pirfenidone should be used with caution in patients with moderate renal impairment (creatinine clearance 30–50 ml/min) and should not be used in patients with severe renal impairment (creatinine clearance < 30 ml/min) or end-stage renal disease requiring dialysis [22]. Renal events are not listed as an adverse reaction of interest with pirfenidone in the product labels [22, 23]. With nintedanib, the European product label states that patients should be monitored during therapy, with particular attention to those patients exhibiting risk factors for renal impairment/failure [22]. In case of renal impairment/failure, therapy adjustment should be considered [22]. The pharmacokinetics of nintedanib have not been studied in patients with severe renal impairment or end-stage renal disease requiring dialysis [24]. Physicians should also be aware that hepatic impairment is a further special consideration when managing patients with IPF on antifibrotics [21,22,23,24].
Other Medications for Patients with IPF
Corticosteroids should not be used in IPF, with the exception that they can be considered in patients experiencing an acute worsening of their condition (acute exacerbation) [11]. International guidelines for the treatment of IPF recommend against the use of long-term corticosteroid therapy [11]. Triple therapy with a combination of prednisone, azathioprine, and N-acetylcysteine was investigated in the PANTHER-IPF (NCT00650091) study in patients with mild-to-moderate lung-function impairment [105]. An interim analysis revealed that there was an increased risk of death and hospitalization in patients receiving triple therapy compared with placebo. This resulted in early termination of the trial, and provided compelling evidence against the combined use of corticosteroids and immunosuppressants in patients with IPF [105].
Cardiovascular Medications in Patients with Comorbid IPF and CVD
When treating patients with comorbid IPF and CVD, it is important to take a holistic approach that keeps in mind the potential burden of polypharmacy in this population [39, 46, 50, 106]. A high proportion of patients with IPF are prescribed statins for cardiovascular indications [107]. In an analysis of data from 22,941 patients with ILD, including 5915 with IPF, in the national Danish Patients Registry, statins were associated with a reduced risk of mortality in patients with ILD and IPF [108]. Similarly, in a post hoc analysis of data from 624 patients randomized to placebo in the pivotal Phase III trials of pirfenidone, patients receiving statins at baseline had a significantly lower risk over 52 weeks for death or 6MWD decline, all-cause and respiratory-related hospitalization, and IPF-related mortality versus patients who were not receiving statins [107]. It should be noted that this post hoc analysis did not investigate outcomes in patients enrolled in the pirfenidone arms of the ASCEND and CAPACITY trials [107]. In a post hoc analysis of patients enrolled in the INPULSIS trials, there was a reduction in annual adjusted FVC decline in patients treated with statins compared with non-users, in both the nintedanib arm and the placebo arm [109]. The treatment effect of nintedanib versus placebo appeared to be consistent between patients who were treated with statins and those who were not [109]. Overall, in the absence of randomized controlled trials investigating statins in patients with IPF, the available evidence suggests that statins might have favorable effects on outcomes in IPF.
In addition to statins, anticoagulants are also commonly prescribed in patients with IPF for comorbid conditions such as atrial fibrillation and venous thromboembolic disease [110]. Theories regarding a potential link between thrombosis and lung fibrosis have previously led to suggestions that anticoagulants could have a role in the treatment of IPF [68]. However, treatment guidelines recommend against the use of anticoagulants for the treatment of IPF, based on concerns regarding the benefit–risk profile [68]. For example, the efficacy and safety of warfarin for treatment of IPF was investigated in the placebo-controlled ACE-IPF trial where 145 patients were randomized to receive warfarin or placebo. Due to a low probability of benefit and a significantly higher mortality rate in patients receiving warfarin versus placebo (14 deaths vs. 3 deaths; p = 0.005), the study was terminated early [111].
In a post hoc analysis of patients enrolled in the placebo arms of the ASCEND and CAPACITY trials, 32 patients who were treated with anticoagulants at baseline (91% of whom received warfarin) for conditions other than IPF were at a higher risk of IPF-related mortality over 52 weeks compared with non-users [112]. The risk of bleeding and cardiac events did not appear to differ between groups [112]. Based on the available evidence, individual risk assessments should be performed for each patient with IPF requiring anticoagulation, and post-marketing safety data on antifibrotics may prove useful at this point [88, 113]. There is obviously an unmet need for future clinical studies to investigate the use of anticoagulants other than warfarin in patients with IPF.
The relationship between the use of other cardiovascular medications and outcomes in patients with IPF has also been investigated in an analysis of data from 272 patients treated at a tertiary referral center for ILD [50]. Predicted survival was not significantly different in treated versus untreated patients for antiplatelet therapy, anticoagulants, beta blockers, statins, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and other antihypertensive drugs [50]. Further clinical research is required to investigate the effects of comorbidities and their treatments on outcomes in IPF, including lung function, QoL, and survival.
Lung Transplantation
LTx has been shown to provide a survival benefit in patients with IPF, with a median post-transplant survival of 4.5 years [114]. International Guidelines for the Selection of Lung Transplant Candidates suggest that patients with IPF might be considered for LTx if they meet the following criteria: ≥ 10% FVC decline during a 6-month period, ≥ 15% DLco decline during a 6-month period, desaturation to < 88% or distance < 250 m during a 6 min walk test or > 50 m decline in 6MWD during a 6-month period, PH on right-heart catheterization or echocardiography, or hospitalization due to respiratory decline, pneumothorax, or acute exacerbation [28]. Potential candidates for LTx with IPF should be referred to a cardiologist as part of their pre-transplant workup. Guidelines for the selection of LTx candidates state that CAD not amenable to revascularization is an absolute contra-indication for LTx [28]. However, a registry study utilizing coronary angiography data from 644 patients who received LTx revealed that 324 patients had CAD, and that there was no difference in mortality between those with and without CAD [115]. Whilst the population of this study was screened, it does suggest that careful evaluation and treatment can allow for selected patients with CAD, including those requiring revascularization, to successfully undergo LTx.
Conclusion
In conclusion, both CVD and IPF share a number of risk factors and affect a similar patient demographic [53]. In addition, some of the signs and symptoms of HF are shared with IPF [14, 16]. In combination with the high prevalence of CVD in patients with IPF, these factors mean that cardiologists may be in a privileged position to identify patients with possible IPF and refer them to a specialist in ILD. The high burden of CVD in patients with IPF also means that cardiologists may have an important role in co-managing affected patients [10, 46]. This includes assessing patients for PH, one of the most frequent cardiovascular complications in patients with IPF [52, 54,55,56, 116].
Increased knowledge among cardiologists regarding the identification and diagnosis of patients with IPF will help to facilitate the diagnosis and treatment of these patients. In addition, increased knowledge among cardiologists regarding the management of patients with comorbid IPF and CVD will help to promote the holistic and multidisciplinary treatment of patients with both conditions.
References
Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–68.
Dalleywater W, Powell HA, Hubbard RB, Navaratnam V. Risk factors for cardiovascular disease in people with idiopathic pulmonary fibrosis: a population-based study. Chest. 2015;147(1):150–6.
Charoenpong P. Risk of coronary artery disease in patients with idiopathic pulmonary fibrosis: a systemic review and meta-analysis. Am J Respir Crit Care Med. 2017;195:A5430.
Broder M, Change E, Papoyan E, et al. Risk of cardiovascular comorbidities in patients with idiopathic pulmonary fibrosis: analysis of Medicare data. Eur Respir J. 2016;48(Suppl 60):PA4919.
Suzuki A, Kondoh Y. The clinical impact of major comorbidities on idiopathic pulmonary fibrosis. Respir Investig. 2017;55(2):94–103.
Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8.
American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International Consensus Statement. Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–64.
Burke GM, Genuardi M, Shappell H, D’Agostino RB Sr, Magnani JW. Temporal associations between smoking and cardiovascular disease, 1971 to 2006 (from the Framingham Heart Study). Am J Cardiol. 2017;120(10):1787–91.
Joseph P, Leong D, McKee M, et al. Reducing the global burden of cardiovascular disease, Part 1: the epidemiology and risk factors. Circ Res. 2017;121(6):677–94.
Jovanovic DM, Mogulkoc N, Sterclova M, et al. Analysis of comorbid conditions in 1210 IPF patients from the EMPIRE registry. Eur Res J. 2017;50(Suppl 61):OA1953.
Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
Berliner D, Schneider N, Welte T, Bauersachs J. The differential diagnosis of dyspnea. Dtsch Arztebl Int. 2016;113(49):834–45.
Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71(8):1529–37.
Collard HR, Tino G, Noble PW, et al. Patient experiences with pulmonary fibrosis. Respir Med. 2007;101(6):1350–4.
Hewson T, McKeever T, Gibson J, Hubbard R, Hutchinson J. Onset of symptoms in idiopathic pulmonary fibrosis: a case–control study. Eur Res J. 2016;48(Suppl 60):PA788.
Cosgrove GP, Bianchi P, Danese S, Lederer DJ. Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey. BMC Pulm Med. 2018;18(1):9.
Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40.
Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21(126):355–61.
Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795–806.
Harari S, Madotto F, Caminati A, Conti S, Cesana G. Epidemiology of idiopathic pulmonary fibrosis in Northern Italy. PLoS ONE. 2016;11(2):e0147072.
European Medicines Agency. Summary of Product Characteristics - Ofev (nintedanib). http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003821/human_med_001834.jsp&mid=WC0b01ac058001d124 (2018). Accessed 01 Nov 2018.
European Medicines Agency. Summary of Product Characteristics - Esbriet (pirfenidone). http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002154/human_med_001417.jsp&mid=WC0b01ac058001d124 (2018). Accessed 10 Sept 2018.
Food and Drug Administration. Highlights of Prescribing Information - Esbriet. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208780s000lbl.pdf (2017). Accessed 10 Sept 2018.
Food and Drug Administration. Highlights of Prescribing Information - Ofev. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205832s009lbl.pdf (2018). Accessed 10 Sept 2018.
Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82.
Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–53.
George TJ, Arnaoutakis GJ, Shah AS. Lung transplant in idiopathic pulmonary fibrosis. Arch Surg. 2011;146(10):1204–9.
Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15.
Bonella F, Wijsenbeek M, Molina-Molina M, et al. European idiopathic pulmonary fibrosis patient charter: a missed opportunity. Eur Respir J. 2016;48(1):283–4.
Bianchi P, Lederer DJ, Danese S, Loboda J, Cosgrove G. Interstitial Lung Disease Patient Diagnostic Journey (INTENSITY) Survey. Am J Respir Crit Care Med. 2016;193:A7894.
Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842–7.
Purokivi M, Hodgson U, Myllärniemi M, Salomaa ER, Kaarteenaho R. Are physicians in primary health care able to recognize pulmonary fibrosis? Eur Clin Respir J. 2017;4(1):1290339.
Vašáková M, Mogulkoc N, Šterclová M, et al. Does timeliness of diagnosis influence survival and treatment response in idiopathic pulmonary fibrosis? Real-world results from the EMPIRE registry. Eur Res J. 2017;50(Suppl 61):PA4880.
Luppi F, Cerri S, Taddei S, Ferrara G, Cottin V. Acute exacerbation of idiopathic pulmonary fibrosis: a clinical review. Intern Emerg Med. 2015;10(4):401–11.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Cottin V, Cordier JF. Velcro crackles: the key for early diagnosis of idiopathic pulmonary fibrosis? Eur Respir J. 2012;40(3):519–21.
Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–61.
Song JW, Song JK, Kim DS. Echocardiography and brain natriuretic peptide as prognostic indicators in idiopathic pulmonary fibrosis. Respir Med. 2009;103(2):180–6.
King CS, Nathan SD. Idiopathic pulmonary fibrosis: effects and optimal management of comorbidities. Lancet Respir Med. 2017;5(1):72–84.
Richeldi L, Ryerson CJ, Lee JS, et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax. 2012;67(5):407–11.
Kawakami R, Nakada Y, Ueda T, Kawata H, Okura H, Saito Y. Pulmonary dysfunction at spirometry and prognosis in patients with acute decompensated heart failure. J Card Fail. 2016;22(9):S209.
Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail. 2015;17(11):1161–71.
Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27(3):595–615.
Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–53.
Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, Spagnolo P. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev. 2014;23(133):308–19.
Kärkkäinen M, Kettunen HP, Nurmi H, Selander T, Purokivi M, Kaarteenaho R. Effect of smoking and comorbidities on survival in idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):160.
Dalleywater W, Powell HA, Fogarty AW, Hubbard RB, Navaratnam V. Venous thromboembolism in people with idiopathic pulmonary fibrosis: a population-based study. Eur Respir J. 2014;44(6):1714–5.
Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277–84.
King TE Jr, Albera C, Bradford WZ, et al. All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med. 2014;189(7):825–31.
Kreuter M, Ehlers-Tenenbaum S, Palmowski K, et al. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS ONE. 2016;11(3):e0151425.
Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41.
Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30(4):715–21.
Oldham JM, Collard HR. Comorbid conditions in idiopathic pulmonary fibrosis: recognition and management. Front Med (Lausanne). 2017;4:123.
Nathan SD, Shlobin OA, Ahmad S, Urbanek S, Barnett SD. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest. 2007;131(3):657–63.
Kimura M, Taniguchi H, Kondoh Y, et al. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration. 2013;85(6):456–63.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119.
Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–9.
Hayes D Jr, Black SM, Tobias JD, Kirkby S, Mansour HM, Whitson BA. Influence of pulmonary hypertension on patients with idiopathic pulmonary fibrosis awaiting lung transplantation. Ann Thorac Surg. 2016;101(1):246–52.
Adir Y, Harari S. Pulmonary hypertension associated with chronic obstructive lung disease and idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2014;20(5):414–20.
Caminati A, Cassandro R, Harari S. Pulmonary hypertension in chronic interstitial lung diseases. Eur Respir Rev. 2013;22(129):292–301.
Farkas L, Kolb M. Pulmonary microcirculation in interstitial lung disease. Proc Am Thorac Soc. 2011;8(6):516–21.
Collum SD, Chen NY, Hernandez AM, et al. Inhibition of hyaluronan synthesis attenuates pulmonary hypertension associated with lung fibrosis. Br J Pharmacol. 2017;174(19):3284–301.
Scotton CJ, Krupiczojc MA, Konigshoff M, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119(9):2550–63.
Wygrecka M, Kwapiszewska G, Jablonska E, et al. Role of protease-activated receptor-2 in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(12):1703–14.
Sode BF, Dahl M, Nielsen SF, Nordestgaard BG. Venous thromboembolism and risk of idiopathic interstitial pneumonia: a nationwide study. Am J Respir Crit Care Med. 2010;181(10):1085–92.
Agrawal A, Verma I, Shah V, Agarwal A, Sikachi RR. Cardiac manifestations of idiopathic pulmonary fibrosis. Intractable Rare Dis Res. 2016;5(2):70–5.
Andersen C, Mellemkjaer S, Hilberg O, Bendstrup E. NT-proBNP < 95 ng/l can exclude pulmonary hypertension on echocardiography at diagnostic workup in patients with interstitial lung disease. Eur Clin Respir J. 2016;3:32027.
Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192(2):e3–19.
Nathan S, Behr J, Collard HR, et al. RISE-IIP: Riociguat for the treatment of pulmonary hypertension associated with idiopathic interstitial pneumonia. Eur Respir J. 2017;50(Suppl 61):OA1985.
Idiopathic Pulmonary Fibrosis Clinical Research Network, Zisman DA, Schwarz M, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8.
ClinicalTrials.gov. Efficacy, Safety, and Tolerability Study of Pirfenidone in Combination With Sildenafil in Participants With Advanced Idiopathic Pulmonary Fibrosis (IPF) and Risk of Group 3 Pulmonary Hypertension. https://clinicaltrials.gov/ct2/show/NCT02951429 (2017). Accessed 10 Sept 2018.
ClinicalTrials.gov. Efficacy and Safety of Nintedanib When Co-administered With Sildenafil in Idiopathic Pulmonary Fibrosis Patients With Advanced Lung Function Impairment. https://clinicaltrials.gov/ct2/show/NCT02802345 (2017). Accessed 10 Sept 2018.
Behr J, Nathan SD, Harari S, et al. Sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: A Phase IIb, randomised, double-blind, placebo-controlled study—Rationale and study design. Respir Med. 2018;138:13–20.
Kolb M, Raghu G, Wells AU, et al. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2018;379(18):1722–31.
Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46(4):1113–30.
Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–42.
Lee JS. The role of gastroesophageal reflux and microaspiration in idiopathic pulmonary fibrosis. Clin Pulm Med. 2014;21(2):81–5.
Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1(5):369–76.
Kreuter M, Wuyts W, Renzoni E, et al. Antacid therapy and disease outcomes in idiopathic pulmonary fibrosis: a pooled analysis. Lancet Respir Med. 2016;4(5):381–9.
ClinicalTrials.gov. Pilot Trial of Omeprazole in Idiopathic Pulmonary Fibrosis (IPF) (PPIPF). https://clinicaltrials.gov/ct2/show/NCT02085018 (2014). Accessed 11 Sept 2018.
Kolilekas L, Manali E, Vlami KA, et al. Sleep oxygen desaturation predicts survival in idiopathic pulmonary fibrosis. J Clin Sleep Med. 2013;9(6):593–601.
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20(120):85–97.
Wollin L, Wex E, Pautsch A, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1434–45.
Nathan SD, Albera C, Bradford WZ, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir Med. 2017;5(1):33–41.
Richeldi L, Cottin V, du Bois RM, et al. Nintedanib in patients with idiopathic pulmonary fibrosis: Combined evidence from the TOMORROW and INPULSIS(®) trials. Respir Med. 2016;113:74–9.
Lancaster L, Albera C, Bradford WZ, et al. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: integrated analysis of cumulative data from 5 clinical trials. BMJ Open Respir Res. 2016;3(1):e000105.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–95.
Noth I, Oelberg D, Kaul M, Conoscenti CS, Raghu G. Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis in the USA. Eur Respir J. 2018;52(1):1702106.
Semeniuk-Wojtaś A, Lubas A, Stec R, Szczylik C, Niemczyk S. Influence of tyrosine kinase inhibitors on hypertension and nephrotoxicity in metastatic renal cell cancer patients. Int J Mol Sci. 2016;17(12):E2073.
Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep. 2012;14(4):285–94.
Glassberg MK, Nathan SD, Lin C-Y, et al. Cardiovascular events in phase 3 trials of pirfenidone in idiopathic pulmonary fibrosis (IPF). Am J Respir Crit Care Med. 2016;193:A4980.
Glassberg MK, Lew C, Raimundo K, et al. Cardiovascular risk factors, comorbidities and concomitant medications from three phase 3 trials of pirfenidone in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:A4979.
Hughes G, Toellner H, Morris H, Leonard C, Chaudhuri N. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med. 2016;5(9):E78.
Chaudhuri N, Duck A, Frank R, Holme J, Leonard C. Real world experiences: pirfenidone is well tolerated in patients with idiopathic pulmonary fibrosis. Respir Med. 2014;108(1):224–6.
Cottin V, Koschel D, Günther A, et al. Long-term safety of pirfenidone in a real-world setting: final results from the prospective, observational PASSPORT registry. Eur Respir J. 2017;50(Suppl 61):PA2806.
Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, Criner GJ. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology. 2017;22(6):1171–8.
Oltmanns U, Kahn N, Palmowski K, et al. Pirfenidone in idiopathic pulmonary fibrosis: real-life experience from a German tertiary referral center for interstitial lung diseases. Respiration. 2014;88(3):199–207.
Cottin V. The safety and tolerability of nintedanib in the treatment of idiopathic pulmonary fibrosis. Expert Opin Drug Saf. 2017;16(7):857–65.
Harari S, Caminati A. Idiopathic pulmonary fibrosis: from clinical trials to real-life experiences. Eur Respir Rev. 2015;24(137):420–7.
Harari S, Elia D, Humbert M. Pulmonary hypertension in parenchymal lung diseases: any future for new therapies? Chest. 2018;153(1):217–23.
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–30.
Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47(10):1987–96.
King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92.
Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9.
Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77.
Reed RM, Eberlein M, Girgis RE, et al. Coronary artery disease is under-diagnosed and under-treated in advanced lung disease. Am J Med. 2012;125(12):1228.e13–22.
Kreuter M, Bonella F, Maher TM, et al. Effect of statins on disease-related outcomes in patients with idiopathic pulmonary fibrosis. Thorax. 2017;72(2):148–53.
Vedel-Krogh S, Nielsen SF, Nordestgaard BG. Statin use is associated with reduced mortality in patients with interstitial lung disease. PLoS ONE. 2015;10(10):e0140571.
Kreuter M, Costabel U, Richeldi L, et al. Statin therapy and outcomes in trials of nintedanib in idiopathic pulmonary fibrosis. Respiration. 2018;95(5):317–26.
Behr J, Kreuter M, Hoeper MM, et al. Management of patients with idiopathic pulmonary fibrosis in clinical practice: the INSIGHTS-IPF registry. Eur Respir J. 2015;46(1):186–96.
Noth I, Anstrom KJ, Calvert SB, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186(1):88–95.
Kreuter M, Wijsenbeek MS, Vasakova M, et al. Unfavourable effects of medically indicated oral anticoagulants on survival in idiopathic pulmonary fibrosis. Eur Respir J. 2016;47(6):1776–84.
Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: design of a double-blind, randomised, placebo-controlled phase II trial. BMJ Open Respir Res. 2018;5(1):e000289.
Kistler KD, Nalysnyk L, Rotella P, Esser D. Lung transplantation in idiopathic pulmonary fibrosis: a systematic review of the literature. BMC Pulm Med. 2014;14:139.
Khandhar SJ, Althouse AD, Mulukutla S, et al. Postoperative outcomes and management strategies for coronary artery disease in patients in need of a lung transplantation. Clin Transplant. 2017;31(9):e13026.
Knight AK, Neto JEDS, Neftelinov ST, Tansey S. Idiopathic pulmonary fibrosis (IPF) co-morbidities and treatment in a global survey. Am J Respir Crit Care Med. 2017;195:A1569.
ClinicalTrials.gov. ARTEMIS-PH—Study of Ambrisentan in Subjects with Pulmonary Hypertension Associated with Idiopathic Pulmonary Fibrosis (ARTEMIS-PH). https://clinicaltrials.gov/ct2/show/NCT00879229 (2014). Accessed 11 Sept 2018.
Raghu G, Behr J, Brown KK, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641–9.
Corte TJ, Keir GJ, Dimopoulos K, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2014;190(2):208–17.
Han MK, Bach DS, Hagan PG, et al. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013;143(6):1699–708.
American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304.
Ryerson CJ, Collard HR. Update on the diagnosis and classification of ILD. Curr Opin Pulm Med. 2013;19(5):453–9.
Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48.
Acknowledgements
Funding
This review was sponsored by F. Hoffmann-La Roche, Ltd. The sponsor is also funding the journal’s article processing charges and Open Access fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing Assistance
Medical writing support was provided by Catherine Stanton of CMC AFFINITY, a division of Complete Medical Communications, Ltd., Glasgow, UK, funded by F. Hoffmann-La Roche, Ltd.
Disclosures
Johan van Cleemput and Andrea Sonaglioni have nothing to disclose. Monica Bengus is a full-time employee of F. Hoffmann La-Roche, Ltd./Genentech, Inc. John L. Stauffer is a full-time employee of F. Hoffmann La-Roche, Ltd./Genentech, Inc. Wim A. Wuyts is on the speakers’ bureau for F. Hoffmann-La Roche, Ltd. and Boehringer Ingelheim. His institution has received funding from both companies. Sergio Harari reports personal fees from Roche, grants and personal fees from Actelion and Boehringer Ingelheim.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7406171.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
van Cleemput, J., Sonaglioni, A., Wuyts, W.A. et al. Idiopathic Pulmonary Fibrosis for Cardiologists: Differential Diagnosis, Cardiovascular Comorbidities, and Patient Management. Adv Ther 36, 298–317 (2019). https://doi.org/10.1007/s12325-018-0857-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0857-z




