Abstract
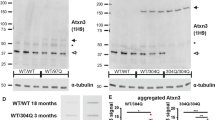
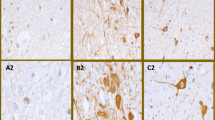
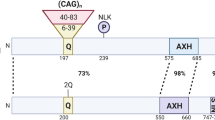
Spinocerebellar ataxia (SCA) is a hereditary neurodegenerative disease. We have generated SCA17 transgenic mice bearing human TBP with 109 CAG repeats under the Purkinje cell-specific L7/pcp2 promoter. These mice recapitulate the patients’ phenotypes and are suitable for the study of the SCA17 pathomechanism. Magnetic resonance imaging (MRI) and immunostainings were performed to identify the neuroimaging spectrum during disease progression. The results indicate that despite an overall normal appearance at birth, postnatal brain damage takes place rapidly in SCA17. Cerebellar atrophy, fourth-ventricle enlargement, and reduced cerebellar N-acetylaspartate levels were detected at the presymptomatic stage, when the mice were juvenile. The aberrations, which included reductions in body weight; cerebral size; striatal size; and the mean, radial, and axial diffusivities of the cerebellum, became more salient as the disease progressed to the old, late-symptomatic stage. Phosphorylated H2A histone family, member X (γH2AX) immunostaining revealed that the cerebellum underwent severe cell senescence in the old stage while the striatum appeared relatively unaffected by aging. Morphometric analysis indicated that the cerebellar atrophy occurred in all subregions with aging. The data establish that the SCA17 mouse brain appears normal at birth but becomes aberrant at the presymptomatic/juvenile stage. More widespread deficits add to the pathological spectrum at the old stage. The study provides information for the expression and expansion of L7/pcp2 promoter and implies the disease progression of SCA17 patients.







Similar content being viewed by others
References
Koide R, Kobayashi S, Shimohata T, Ikeuchi T, Maruyama M, Saito M, et al. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease? Hum Mol Genet. 1999;8:2047–53.
Rolfs A, Koeppen AH, Bauer I, Bauer P, Buhlmann S, Topka H, et al. Clinical features and neuropathology of autosomal dominant spinocerebellar ataxia (SCA17). Ann Neurol. 2003;54:367–75.
van Roon-Mom WM, Reid SJ, Faull RL, Snell RG. TATA-binding protein in neurodegenerative disease. Neuroscience. 2005;133:863–72.
Toyoshima Y, Yamada M, Onodera O, Shimohata M, Inenaga C, Fujita N, et al. SCA17 homozygote showing Huntington’s disease-like phenotype. Ann Neurol. 2004;55:281–6.
Bruni AC, Takahashi-Fujigasaki J, Maltecca F, Foncin JF, Servadio A, Casari G, et al. Behavioral disorder, dementia, ataxia, and rigidity in a large family with TATA box-binding protein mutation. Arch Neurol. 2004;61:1314–20.
Nanda A, Jackson SA, Schwankhaus JD, Metzer WS. Case of spinocerebellar ataxia type 17 (SCA17) associated with only 41 repeats of the TATA-binding protein (TBP) gene. Mov Disord. 2007;22:436.
Ali KW, Robertson NP. Lessons from preclinical disease. J Neurol. 2013;260:2193–5.
Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72.
Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5.
Chang YC, Lin CY, Hsu CM, Lin HC, Chen YH, Lee-Chen GJ, et al. Neuroprotective effects of granulocyte-colony stimulating factor in a novel transgenic mouse model of SCA17. J Neurochem. 2011;118:288–303.
Cui Y, Yang S, Li XJ, Li S. Genetically modified rodent models of SCA17. J Neurosci Res. 2017;95:1540–7.
Figiel M, Szlachcic WJ, Switonski PM, Gabka A, Krzyzosiak WJ. Mouse models of polyglutamine diseases: review and data table. Part I Mol Neurobiol. 2012;46:393–429.
Huang S, Ling JJ, Yang S, Li XJ, Li S. Neuronal expression of TATA box-binding protein containing expanded polyglutamine in knock-in mice reduces chaperone protein response by impairing the function of nuclear factor-Y transcription factor. Brain. 2011;134:1943–58.
Kelp A, Koeppen AH, Petrasch-Parwez E, Calaminus C, Bauer C, Portal E, et al. A novel transgenic rat model for spinocerebellar ataxia type 17 recapitulates neuropathological changes and supplies in vivo imaging biomarkers. J Neurosci. 2013;33:9068–81.
Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13:159–63.
Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407.
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21.
Garden GA, Libby RT, Fu YH, Kinoshita Y, Huang J, Possin DE, et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous Purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci. 2002;22:4897–905.
Minnerop M, Joe A, Lutz M, Bauer P, Urbach H, Helmstaedter C, et al. Putamen dopamine transporter and glucose metabolism are reduced in SCA17. Ann Neurol. 2005;58:490–1.
Brockmann K, Reimold M, Globas C, Hauser TK, Walter U, Machulla HJ, et al. PET and MRI reveal early evidence of neurodegeneration in spinocerebellar ataxia type 17. J Nucl Med. 2012;53:1074–80.
Chen CC, Tung YY, Chang C. A lifespan MRI evaluation of ventricular enlargement in normal aging mice. Neurobiol Aging. 2011;32:2299–307.
Miller MI, Trouve A, Younes L. On the metrics and Euler-Lagrange equations of computational anatomy. Annu Rev Biomed Eng. 2002;4:375–405.
Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6:93–108.
Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–86.
Enokido Y, Yoshitake A, Ito H, Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun. 2008;376:128–33.
Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004.
Allan R, Chaseling R, Graf N, Dexter M. Aqueduct stenosis-?Benign. J Clin Neurosci. 2005;12:178–82.
Trottier Y, Lutz Y, Stevanin G, Imbert G, Devys D, Cancel G, et al. Polyglutamine expansion as a pathological epitope in Huntington’s disease and four dominant cerebellar ataxias. Nature. 1995;378:403–6.
Klein FA, Zeder-Lutz G, Cousido-Siah A, Mitschler A, Katz A, Eberling P, et al. Linear and extended: a common polyglutamine conformation recognized by the three antibodies MW1, 1C2 and 3B5H10. Hum Mol Genet. 2013;22:4215–23.
Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–22.
Reetz K, Kleiman A, Klein C, Lencer R, Zuehlke C, Brockmann K, et al. CAG repeats determine brain atrophy in spinocerebellar ataxia 17: a VBM study. PLoS One. 2011;6:e15125.
Maltecca F, Filla A, Castaldo I, Coppola G, Fragassi NA, Carella M, et al. Intergenerational instability and marked anticipation in SCA-17. Neurology. 2003;61:1441–3.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Funding
We received financial support from Ministry of Science and Technology, Taiwan, 102-2321-B-001-062, 103-2321-B-001-043, 105-2325-B-003-003, and 108-2320-B-003-005.
Author information
Authors and Affiliations
Contributions
Chiao-Chi Chen, Ph.D., designed the study, conducted data interpretation, and contributed to the writing; Nai-Wei Yao performed the MRI experiment and conducted data analysis; Nai-Wei Yao and Chia-Wei Lin performed the immunostaining experiment and conducted data analysis; Wei-Shuo Su and Chin-Tien Wu conducted LDDMM; Chen Chang, Ph.D., designed the MRI study and contributed to the writing; Hsiu Mei Hsieh-Li, Ph.D., designed the immunofluorescent staining experiment, and contributed to the writing.
Corresponding authors
Ethics declarations
All of the experimental procedures performed in this study were approved by the Institutional Animal Care and Usage Committee of Academia Sinica and National Taiwan Normal University (No. 104010).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, CC., Yao, NW., Lin, CW. et al. Neuroimaging Spectrum at Pre-, Early, and Late Symptomatic Stages of SCA17 Mice. Cerebellum 19, 487–500 (2020). https://doi.org/10.1007/s12311-020-01127-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01127-5