Abstract
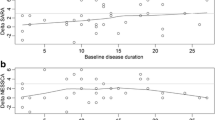
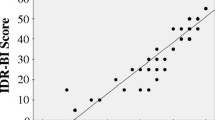
Spinocerebellar ataxia type 2 (SCA2), caused by a CAG expansion (CAGexp) at ATXN2, has a complex clinical picture. While validated ataxia scales are available, comprehensive instruments to measure all SCA2 neurological manifestations are required. This study aims to validate the Neurological Examination Score for the assessment of Spinocerebellar Ataxias (NESSCA) to be used in SCA2 and to compare its responsiveness to those obtained with other instruments. NESSCA, SARA, SCAFI, and CCFS scales were applied in symptomatic SCA2 patients. Correlations were done with age at onset, disease duration, CAGexp, and between scales. Responsiveness was estimated by comparing deltas of stable to worse patients after 12 months, according to Patient Global Impression of change, and the area under the curve (AUC) of the Receiver Operating Characteristics curve of scores range. Eighty-eight evaluations (49 patients) were obtained. NESSCA had an even distribution and correlated with disease duration (r = 0.55), SARA (r = 0.63), and CAGexp (rho = 0.32): both explained 44% of NESSCA variance. Deltas (95% CI) after 1 year in stable and worse patients were only significantly different for SARA. NESSCA, SARA, SCAFI, and CCFS AUC were 0.63, 0.81, 0.49, and 0.48, respectively. NESSCA is valid to be used in SCA2. However, the only instrument that presented good responsiveness to change in 1 year was SARA. We suggest that NESSCA can be used as a secondary outcome in future trials in SCA2 due to the burden of neurological disabilities related to disease progression.



Similar content being viewed by others
References
Velázquez-Pérez L, Rodríguez-Labrada R, García-Rodríguez JC, Almaguer-Mederos LE, Cruz-Mariño T, Laffita-Mesa JM. A comprehensive review of spinocerebellar ataxia type 2 in Cuba. Cerebellum. 2011;10(2):184–98.
Pulst SM. Spinocerebellar Ataxia Type 2. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993–2016. 1998.
Auburger GWJ. Spinocerebellar ataxia type 2. In: Subramony SH, Dürr A, editors. Handbook of clinical neurology: ataxic disorders. Edinburgh: Elsevier; 2012. p. 423–36.
Sequeiros J, Martins S, Silveira I. Epidemiology and population genetics of degenerative ataxias. Handb Clin Neurol. 2012;103:227–51.
de Castilhos RM, Furtado GV, Gheno TC, Schaeffer P, Russo A, Barsottini O, Pedroso JL, Salarini DZ, Vargas FR, de Lima MA, Godeiro C, Santana-da-Silva LC, Toralles MB, Santos S, van der Linden Jr H, Wanderley HY, de Medeiros PF, Pereira ET, Ribeiro E, Saraiva-Pereira ML, Jardim LB, Rede Neurogenetica. Spinocerebellar ataxias in Brazil—frequencies and modulating effects of related genes. Cerebellum. 2014;13(1):17–28.
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schöls L, Szymanski S, van de Warrenburg BP, Dürr A, Klockgether T, Fancellu R. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Schmitz-Hübsch T, Giunti P, Stephenson DA, Globas C, Baliko L, Saccà F, Mariotti C, Rakowicz M, Szymanski S, Infante J, van de Warrenburg BP, Timmann D, Fancellu R, Rola R, Depondt C, Schöls L, Zdzienicka E, Kang JS, Döhlinger S, Kremer B, Melegh B, Filla A, Klockgether T. SCA functional index: a useful compound performance measure for spinocerebellar ataxia. Neurology. 2008a;71(7):486–92.
du Montcel ST, Charles P, Ribai P, Goizet C, Le Bayon A, Labauge P, Guyant-Maréchal L, Forlani S, Jauffret C, Vandenberghe N, N’guyen K, Le Ber I, Devos D, Vincitorio CM, Manto MU, Tison F, Hannequin D, Ruberg M, Brice A, Durr A. Composite cerebellar functional severity score: validation of a quantitative score of cerebellar impairment. Brain. 2008;131(Pt 5):1352–61.
Schmitz-Hübsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, Filla A, Mariotti C, Rakowicz M, Charles P, Ribai P, Szymanski S, Infante J, van de Warrenburg BP, Dürr A, Timmann D, Boesch S, Fancellu R, Rola R, Depondt C, Schöls L, Zdienicka E, Kang JS, Döhlinger S, Kremer B, Stephenson DA, Melegh B, Pandolfo M, di Donato S, du Montcel ST, Klockgether T. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology. 2008;71(13):982–9.
Kieling C, Rieder CR, Silva AC, Saute JA, Cecchin CR, Monte TL, Jardim LB. A neurological examination score for the assessment of spinocerebellar ataxia 3 (SCA3). Eur J Neurol. 2008;15(4):371–6.
Furr RM, Bacharach VR. Psychometrics: an introduction. Los Angeles: Sage Publications; 2008.
Streiner DL, Norman GR. Health Measurement scales, a practical guide to their development and use. 4th ed. Oxford: Oxford University Press; 2008. p. 247–74.
Rüb U, Schöls L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K, Korf HW, Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol. 2013;104:38–66.
Bürk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, Didierjean O, Brice A, Klockgether T. Autosomal dominant cerebellar ataxia type I. Clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119:1497–505.
Bürk K, Globas C, Bosch S, Gräber S, Abele M, Brice A, Dichgans J, Daum I, Klockgether T. Cognitive deficits in spinocerebellar ataxia 2. Brain. 1999;122:769–77.
Cancel G, Dürr A, Didierjean O, Imbert G, Bürk K, Lezin A, Belal S, Benomar A, Abada-Bendib M, Vial C, Guimaraes J, Chneiweiss A, Stevanin G, Yvert G, Abbas N, Saudou F, Lebre AS, Yahyaoui M, Hentati F, Vernant JC, Klockgether T, Mandel JL, Agid Y, Brice A. Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum Mol Genet. 1997;6:709–15.
Charles P, Camuzat A, Benammar N, Sellal F, Destee A, Bonnet AM, Lesage S, Le Ber I, Stevanin G, Durr A, Brice A, French Parkinson’s Disease Genetic Study Group. Are interrupted SCA2 CAG repeat expansions responsible for parkinsonism? Neurology. 2007;69:1970–5.
Jacobi H, Bauer P, Giunti P, et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology. 2011;77:1035–41.
Klockgether T, Ludtke R, Kramer B, Abele M, Burk K, Schols L, Riess O, Laccone F, Boesch S, Lopes-Cendes I, Brice A, Inzelberg R, Zilber N, Dichgans J. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121:589–600.
Schols L, Amoiridis G, Büttner T, Przuntek H, Epplen JT, Riess O. Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Ann Neurol. 1997;42:924–32.
Storey E, Forrest SM, Shaw JH, Mitchell P, Gardner RJ. Spinocerebellar ataxia type 2: clinical features of a pedigree displaying prominent frontal-executive dysfunction. Arch Neurol. 1999;56:43–50.
Pedroso JL, de Freitas ME, Albuquerque MV, Saraiva-Pereira ML, Jardim LB, Barsottini OG. Should spinocerebellar ataxias be included in the differential diagnosis for Huntington’s diseases-like syndromes? J Neurol Sci. 2014;347(1–2):356–8.
Velázquez-Pérez L, Seifried C, Santos-Falco N, Abele M, Ziemann U, Almaguer LE, Martınez-Gongora E, Sánchez-Cruz G, Canales N, Perez-Gonzalez R, Velazquez-Manresa M, Viebahn B, von Stuckrad-Barre S, Fetter M, Klockgether T, Auburger G. Saccade velocity is controlled by polyglutamine size in spinocerebellar ataxia 2. Ann Neurol. 2004;56:444–7.
Pedroso JL, Braga-Neto P, Escorcio-Bezerra ML, Abrahão A, de Albuquerque MV, Filho FM, de Souza PV, de Rezende Pinto WB, Borges FR Jr, Saraiva-Pereira ML, Jardim LB, Barsottini OG. Non-motor and extracerebellar features in spinocerebellar ataxia type 2. Cerebellum. 2016.
Castilhos RM, Souza AF, Furtado GV, Gheno TC, Silva AL, Vargas FR, Lima MA, Barsottini O, Pedroso JL, Godeiro Jr C, Salarini D, Pereira ET, Lin K, Toralles MB, Saute JA, Rieder CR, Quintas M, Sequeiros J, Alonso I, Saraiva-Pereira ML, Jardim LB. Huntington disease and Huntington disease-like in a case series from Brazil. Clin Genet. 2014;86(4):373–7.
Jacobi H, du Montcel ST, Bauer P, Giunti P, Cook A, Labrum R, Parkinson MH, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, Panzeri M, Rakowicz M, Sulek A, Sobanska A, Schmitz-Hübsch T, Schöls L, Hengel H, Baliko L, Melegh B, Filla A, Antenora A, Infante J, Berciano J, van de Warrenburg BP, Timmann D, Szymanski S, Boesch S, Kang JS, Pandolfo M, Schulz JB, Molho S, Diallo A, Klockgether T. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: a longitudinal cohort study. Lancet Neurol. 2015;14(11):1101–8.
Tezenas du Montcel S, Charles P, Goizet C, Marelli C, Ribai P, Vincitorio C, Anheim M, Guyant-Maréchal L, Le Bayon A, Vandenberghe N, Tchikviladzé M, Devos D, Le Ber I, N’Guyen K, Cazeneuve C, Tallaksen C, Brice A, Durr A. Factors influencing disease progression in autosomal dominant cerebellar ataxia and spastic paraplegia. Arch Neurol. 2012;69(4):500–8.
Jacobi H, Rakowicz M, Rola R, Fancellu R, Mariotti C, Charles P, Dürr A, Küper M, Timmann D, Linnemann C, Schöls L, Kaut O, Schaub C, Filla A, Baliko L, Melegh B, Kang JS, Giunti P, van de Warrenburg BP, Fimmers R, Klockgether T. Inventory of non-ataxia signs (INAS): validation of a new clinical assessment instrument. Cerebellum. 2013;12(3):418–28.
Schmitz-Hübsch T, Fimmers R, Rakowicz M, Rola R, Zdzienicka E, Fancellu R, Mariotti C, Linnemann C, Schöls L, Timmann D, Filla A, Salvatore E, Infante J, Giunti P, Labrum R, Kremer B, van de Warrenburg BP, Baliko L, Melegh B, Depondt C, Schulz J, du Montcel ST, Klockgether T. Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology. 2010;74(8):678–84. doi:10.1212/WNL.0b013e3181d1a6c9.
Chan E, Charles P, Ribai P, Goizet C, Marelli C, Vincitorio CM, Le Bayon A, Guyant-Maréchal L, Vandenberghe N, Anheim M, Devos D, Freeman L, Le Ber I, N’Guyen K, Tchikviladzé M, Labauge P, Hannequin D, Brice A, Durr A, du Montcel ST. Quantitative assessment of the evolution of cerebellar signs in spinocerebellar ataxias. Mov Disord. 2011;26(3):534–8. doi:10.1002/mds.23531.
Acknowledgements
The authors would like to thank the families who agreed to participate in this study. This work was supported by the following Brazilian agencies: CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico—by the Project 78057/2012-1, FIPE-HCPA—Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre—by the Projects GPPG HCPA 12-0357 and 12-0396, and by INAGEMP—Instituto Nacional de Genética Médica Populacional. M.A. and L.D.L.C. were supported by FAPERGS. ERR, MA, ASPS, MLSP, and LBJ were supported by CNPq.
Author information
Authors and Affiliations
Consortia
Contributions
1. Research project: (A) conception, (B) organization, (C) execution. 2. Statistical analysis: (A) design, (B) execution, (C) review and critique. 3. Manuscript preparation: (A) writing of the first draft, (B) review and critique.
T.L.M.: 1 B and C; 2 C; 3 B. E.R.R.: 1 C; 3 B. M.A.: 1 C; 3 B. A.S.P.S.: 1 C; 3 B. L.D.L.C.: 1 C; 3 B. O.B.: 1 C; 3 B. J.L.P.: 1 C; 3 B. F.R.V.: 1 C; 3 B. M.L.S.P.: 1 C; 3 B. V.B.L.T.: 1 B; 2 A, B, and C; 3B. L.B.J.: 1 A and B; 2 A and C; 3 A and B.
Corresponding author
Ethics declarations
Conflict of Interest and Financial Disclosure Related to Research
The authors report no disclosures.
Rights and permissions
About this article
Cite this article
Monte, T.L., Reckziegel, E.R., Augustin, M.C. et al. NESSCA Validation and Responsiveness of Several Rating Scales in Spinocerebellar Ataxia Type 2. Cerebellum 16, 852–858 (2017). https://doi.org/10.1007/s12311-017-0855-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0855-8