Abstract
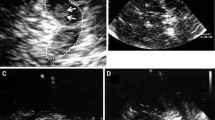
Our objective was to determine whether substantia nigra (SN) hyperechogenicity is greater in spinocerebellar ataxias (SCA) with nigrostriatal affectation than in ataxias without it. A cross-sectional case-control study analyzing four groups of patients was conducted: 1) nigrostriatal ataxias (SCA3 and SCA6), 2) nigrostriatal healthy controls matched by age and sex, 3) non-nigrostriatal ataxias (FRDA and SCA7), and 4) non-nigrostriatal healthy controls matched by age and sex. All the patients underwent a transcranial ultrasound performed by an experienced sonographer blinded to the clinical, genetic, and neuroimaging data. The SN area was measured and compared in the four groups. The SN area was also correlated with clinical features and genetic data in the two ataxia groups. We examined 12 patients with nigrostriatal ataxia (11 SCA3 and 1 SCA6), 12 nigrostriatal healthy control patients, 7 patients with non-nigrostriatal ataxia (5 FRDA and 2 SCA7), and 7 non-nigrostriatal healthy control patients. The median (IQR) SN area (cm2) was greater in the nigrostriatal ataxias compared with the controls (right SN, 0.43 [0.44] vs. 0.11 [0.25]; P = 0.001; left SN, 0.32 [0.25] vs. 0.11 [0.16]; P = 0.001), but was similar among the non-nigrostriatal ataxias and controls. There were no statistically significant differences in the SN area between the nigrostriatal and non-nigrostriatal ataxias, although there was a tendency for a greater left SN area in the nigrostriatal compared with the non-nigrostriatal ataxias (0.32 [0.25] vs. 0.16 [0.24], P = 0.083). SN echogenicity is markedly greater in ataxias with nigrostriatal pathology than in controls. The role of SN hyperechogenicity in differentiating ataxias with and without nigrostriatal pathology should be elucidated in future studies.


Similar content being viewed by others
References
Berg D. Substantia nigra hyperechogenicity is a risk marker or Parkinson’s disease: yes. J Neural Transm. 2011;118:613–9.
Berg D, Grote C, Rausch WD, Maurer M, Wesemann W, Riederer P, et al. Iron accumulation in the substantia nigra in rats visualized by ultrasound. Ultrasound Med Biol. 1999;25:901–4.
Berg D, Roggendorf W, Schroder U, Klein R, Tatschner T, Benz P, et al. Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol. 2002;59:999–1005.
Zecca L, Berg D, Arzberger T, Ruprecht P, Rausch WD, Musicco M, et al. In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov Disord. 2005;20:1278–85.
Berg D, Godau J, Riederer P, Gerlach M, Arzberger T. Microglia activation is related to substantia nigra echogenicity. J Neural Transm. 2010;117:1287–92.
Krogias C, Postert T, Eyding J. Transcranial sonography in ataxia. Int Rev Neurobiol. 2010;90:217–35.
Wolters A, Walter U, Benecke R, Rolfs A. Characterization of autosomal dominant spinocerebellar ataxias with transcranial magnetic stimulation and transcranial brain parenchyma sonography. Kinische Neurophysiol. 2005;36:9–13.
Mijajlović M, Dragasević N, Stefanova E, Petrović I, Svetel M, Kostić VS. Transcranial sonography in spinocerebellar ataxia type 2. J Neurol. 2008;255:1164–7.
Postert T, Eyding J, Berg D, Przuntek H, Becker G, Finger M, et al. Transcranial sonography in spinocerebellar ataxia type 3. J Neural Transm Suppl. 2004;68:123–33.
Pedroso JL, Bor-Seng-Shu E, Felício AC, Braga-Neto P, Teixeira MJ, Barsottini OG. Transcranial sonography findings in spinocerebellar ataxia type 3 (Machado-Joseph disease): a cross-sectional study. Neurosci Lett. 2011;504:98–101.
Furtado S, Farrer M, Tsuboi Y, Klimek ML, de la Fuente-Fernandez R, Hussey J, et al. SCA-2 presenting as parkinsonism in an Alberta family: clinical, genetic, and PET findings. Neurology. 2002;59:1625–7.
Lu CS, Wu Chou YH, Kuo PC, Chang HC, Weng YH. The parkinsonian phenotype of spinocerebellar ataxia type 2. Arch Neurol. 2004;61:35–8.
Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304.
Orozco Diaz G, Nodarse Fleites A, Cordovés Sagaz R, Auburger G. Autosomal dominant cerebellar ataxia: clinical analysis of 263 patients from a homogeneous population in Holguin, Cuba. Neurology. 1990;40:1369–75.
Rub U, de Vos RA, Schultz C, Brunt ER, Paulson H, Braak H. Spinocerebellar ataxia type 3 (Machado–Joseph disease): severe destruction of the lateral reticular nucleus. Brain. 2002;125:2115–24.
Rub U, Del Turco D, Del Tredici K, de Vos RA, Brunt ER, Reifenberger G, et al. Thalamic involvement in a spinocerebellar ataxia type 2 (SCA2) and a spinocerebellar ataxia type 3 (SCA3) patient, and its clinical relevance. Brain. 2003;126:2257–72.
Rub U, Burk K, Schöls L, Brunt ER, de Vos RA, Diaz GO, et al. Damage to the reticulotegmental nucleus of the pons in spinocerebellar ataxia type 1, 2, and 3. Neurology. 2004;63:1258–63.
van Gaalen J, Giunti P, van de Warrenburg BP. Movement disorders in spinocerebellar ataxias. Mov Disord. 2011;26:792–800.
Kim JM, Lee JY, Kim HJ, Kim JS, Kim YK, Park SS, et al. The wide clinical spectrum and nigrostriatal dopaminergic damage in spinocerebellar ataxia type 6. J Neurol Neurosurg Psychiatry. 2010;81:529–32.
Gierga K, Schelhaas HJ, Brunt ER, Seiden K, Scherzed W, Egensperger R, et al. Spinocerebellar ataxia type 6 (SCA6): neurodegeneration goes beyond the known brain predilection sites. Neuropathol Appl Neurobiol. 2009;35:515–27.
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rüb U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21.
Koeppen AH. Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303:1–12.
Lamarche JB, Lemieux B, Lieu HB. The neuropathology of “typical” Friedreich’s ataxia in Quebec. Can J Neurol Sci. 1984;11(4 Suppl):592–600.
Jitpimolmard S, Small J, King RH, Geddes J, Misra P, McLaughlin J, et al. The sensory neuropathy of Friedreich’s ataxia: an autopsy study of a case with prolonged survival. Acta Neuropathol. 1993;86:29–35.
Koeppen AH, Morral JA, McComb RD, Feustel PJ. The neuropathology of late-onset Friedreich’s ataxia. Cerebellum. 2011;10:96–103.
Synofzik M, Godau J, Lindig T, Schöls L, Berg D. Restless legs and substantia nigra hypoechogenicity are common features in Friedreich’s ataxia. Cerebellum. 2011;10:9–13.
Synofzik M, Godau J, Lindig T, Schöls L, Berg D. Transcranial sonography reveals cerebellar, nigral, and forebrain abnormalities in Friedreich’s ataxia. Neurodegener Dis. 2011;8:470–5.
Stocker H, Sojer M, Hering S, Nachbauer W, Seppi K, Schmidauer C, et al. Substantia nigra hypoechogenicity in Friedreich ataxia. Mov Disord. 2012;27:332–3.
Sierra M, Infante J, Berciano J. Substantia nigra echogenicity in Friedreich’s ataxia patients. Cerebellum 2012 Dec 13.
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20.
Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord. 2007;22:2386–93.
Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19.
Folstein M, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Deidel G, et al. Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol. 2007;33:15–25.
Behnke S, Schroeder U, Dillmann U, Buchholz HG, Schreckenberger M, Fuss G, et al. Hyperechogenicity of the substantia nigra in healthy controls is related to MRI changes and to neuronal loss as determined by F-Dopa PET. Neuroimage. 2009;47:1237–43.
Pedroso JL, Bor-Seng-Shu E, Felicio AC, Braga-Neto P, Hoexter MQ, Teixeira MJ, et al. Substantia nigra echogenicity is correlated with nigrostriatal impairment in Machado-Joseph disease. Parkinsonism Relat Disord. 2013;19:742–5.
Pedroso JL, Bor-Seng-Shu E, Felicio AC, Braga-Neto P, Dutra LA, de Aquino CC et al. Severity of restless legs syndrome is inversely correlated with echogenicity of the substantia nigra in different neurodegenerative movement disorders. A preliminary observation. J Neurol Sci 2012;31959–62.
Todd G, Noyes C, Flavel SC, Della Vedova CB, Spyropoulos P, Chatterton B, et al. Illicit stimulant use is associated with abnormal substantia nigra morphology in humans. PLoS One. 2013;8:e56438.
Acknowledgments
The authors thank Juliette Siegfried at ServingMed.com for language editing of the manuscript. Dr. Arpa has received grant funding for other projects from the Agencia Pedro Laín Entralgo (Madrid, Spain) and the Spanish Ministry of Health.
Conflict of Interest
This project has been supported by a grant from the Spanish Ministry of Health, Social Policy and Equality (TRA-052). The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Sánchez, P., Cazorla-García, R., Sanz-Gallego, I. et al. Substantia Nigra Echogenicity in Hereditary Ataxias With and Without Nigrostriatal Pathology: a Pilot Study. Cerebellum 14, 240–246 (2015). https://doi.org/10.1007/s12311-014-0642-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-014-0642-8