Abstract
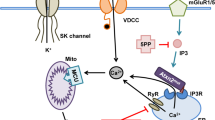
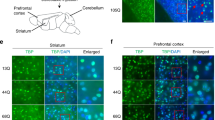
The mutated ataxin-1 protein in spinocerebellar ataxia 1 (SCA1) targets Purkinje cells (PCs) of the cerebellum and causes progressive ataxia due to loss of PCs and neurons of the brainstem. The exact mechanism of this cellular loss is still not clear. Currently, there are no treatments for SCA1; however, understanding of the mechanisms that regulate SCA1 pathology is essential for devising new therapies for SCA1 patients. We previously established a connection between the loss of intracellular calcium-buffering and calcium-signalling proteins with initiation of neurodegeneration in SCA1 transgenic (Tg) mice. Recently, acid-sensing ion channel 1a (ASIC1a) have been implicated in calcium-mediated toxicity in many brain disorders. Here, we report generating SCA1 Tg mice in the ASIC1a knockout (KO) background and demonstrate that the deletion of ASIC1a gene expression causes suppression of the SCA1 disease phenotype. Loss of the ASIC1a channel in SCA1/ASIC1a KO mice resulted in the improvement of motor deficit and decreased PC degeneration. Interestingly, the expression of the ASIC1 variant, ASIC1b, was upregulated in the cerebellum of both SCA1/ASIC1a KO and ASIC1a KO animals as compared to the wild-type (WT) and SCA1 Tg mice. Further, these SCA1/ASIC1a KO mice exhibited translocation of PC calcium-binding protein calbindin-D28k from the nucleus to the cytosol in young animals, which otherwise have both cytosolic and nuclear localization. Furthermore, in addition to higher expression of calcium-buffering protein parvalbumin, PCs of the older SCA1/ASIC1a KO mice showed a decrease in morphologic abnormalities as compared to the age-matched SCA1 animals. Our data suggest that ASIC1a may be a mediator of SCA1 pathogenesis and targeting ASIC1a could be a novel approach to treat SCA1.






Similar content being viewed by others
References
Matilla-Duenas A, Goold R, Giunti P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum. 2008;7(2):106–14.
Matilla-Duenas A et al. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum. 2010;9(2):148–66.
Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci. 2000;23:217–47.
Vig PJ, Subramony SH, McDaniel DO. Calcium homeostasis and spinocerebellar ataxia-1 (SCA-1). Brain Res Bull. 2001;56(3–4):221–5.
Burright EN et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82(6):937–48.
Vig PJ et al. Bergmann glial S100B activates myo-inositol monophosphatase 1 and co-localizes to purkinje cell vacuoles in SCA1 transgenic mice. Cerebellum. 2009;8(3):231–44.
Vig PJ et al. Reduced immunoreactivity to calcium-binding proteins in Purkinje cells precedes onset of ataxia in spinocerebellar ataxia-1 transgenic mice. Neurology. 1998;50(1):106–13.
Vig PJ et al. Suppression of calbindin-D28k expression exacerbates SCA1 phenotype in a disease mouse model. Cerebellum. 2012;11(3):718–32.
Waldmann R et al. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386(6621):173–7.
Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271(14):7879–82.
Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8(3):418–24.
Garcia-Anoveros J et al. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94(4):1459–64.
Lingueglia E et al. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272(47):29778–83.
Chen CC et al. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95(17):10240–5.
Akopian AN et al. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11(10):2217–22.
Grunder S et al. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11(8):1607–11.
Benson CJ et al. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99(4):2338–43.
Sherwood TW et al. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31(26):9723–34.
Jasti J et al. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449(7160):316–23.
Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia-related signals. J Physiol. 2002;543(Pt 2):521–9.
Akaike N, Ueno S. Proton-induced current in neuronal cells. Prog Neurobiol. 1994;43(1):73–83.
Isaev NK et al. Acidosis-induced zinc-dependent death of cultured cerebellar granule neurons. Cell Mol Neurobiol. 2010;30(6):877–83.
Vergo S et al. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134(Pt 2):571–84.
Yuan FL et al. Acid-sensing ion channel 1a mediates acid-induced increases in intracellular calcium in rat articular chondrocytes. Mol Cell Biochem. 2010;340(1–2):153–9.
Xiong ZG et al. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8(1):25–32.
Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels—novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–86.
Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006;209(1):59–68.
Yermolaieva O et al. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101(17):6752–7.
Friese MA et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13(12):1483–9.
Wong HK et al. Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet. 2008;17(20):3223–35.
Hu R et al. Role of acid-sensing ion channel 1a in the secondary damage of traumatic spinal cord injury. Ann Surg. 2011;254(2):353–62.
Shen L et al. Research on screening and identification of proteins interacting with ataxin-3. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22(3):242–7.
Xiong ZG et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118(6):687–98.
Zhang S et al. Cortical GABAergic neurons and cerebellar Purkinje cells respond to ischemia-pathogenic factors differently. Brain Res. 2011;1382:291–7.
Zha XM et al. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103(44):16556–61.
Wemmie JA et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34(3):463–77.
Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29(10):578–86.
McDonald FJ et al. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci U S A. 1999;96(4):1727–31.
Hearst SM et al. Dopamine D2 receptor signaling modulates mutant ataxin-1S776 phosphorylation and aggregation. J Neurochem. 2010;114(3):706–16.
Page AJ et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127(6):1739–47.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Vig PJ et al. Glial S100B protein modulates mutant ataxin-1 aggregation and toxicity: TRTK12 peptide, a potential candidate for SCA1 therapy. Cerebellum. 2011;10(2):254–66.
Clark HB et al. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17(19):7385–95.
Chai S et al. Activation of acid-sensing ion channel 1a (ASIC1a) by surface trafficking. J Biol Chem. 2010;285(17):13002–11.
Bassler EL et al. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276(36):33782–7.
Vig PJ et al. Decreased parvalbumin immunoreactivity in surviving Purkinje cells of patients with spinocerebellar ataxia-1. Neurology. 1996;47(1):249–53.
Vig PJ et al. Relationship between ataxin-1 nuclear inclusions and Purkinje cell specific proteins in SCA-1 transgenic mice. J Neurol Sci. 2000;174(2):100–10.
Vig PJ et al. Glial S100B positive vacuoles in purkinje cells: earliest morphological abnormality in SCA1 transgenic mice. J Neurol Sci Turk. 2006;23(3):166–74.
Cummings CJ et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10(14):1511–8.
Duvick L et al. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 2010;67(6):929–35.
Orr HT et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4(3):221–6.
Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621.
Vig PJ et al. Intranasal administration of IGF-I improves behavior and Purkinje cell pathology in SCA1 mice. Brain Res Bull. 2006;69(5):573–9.
Park J et al. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498(7454):325–31.
Wang YZ et al. Intracellular ASIC1a regulates mitochondrial permeability transition-dependent neuronal death. Cell Death Differ. 2013;20(10):1359–69.
Gao J et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–46.
Duan B et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci. 2011;31(6):2101–12.
Wu LJ et al. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279(42):43716–24.
Baron A et al. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28(6):1498–508.
Hoagland EN et al. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J Biol Chem. 2010;285(53):41852–62.
Jiang Q, Zha XM, Chu XP. Inhibition of human acid-sensing ion channel 1b by zinc. Int J Physiol Pathophysiol Pharmacol. 2012;4(2):84–93.
Diochot S et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490(7421):552–5.
Kasumu A, Bezprozvanny I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum, 2010.
Liu J et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29(29):9148–62.
Chen X et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28(48):12713–24.
Ikeda Y et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38(2):184–90.
Lorenzo DN et al. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J Cell Biol. 2010;189(1):143–58.
Watase K et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci U S A. 2008;105(33):11987–92.
Adachi N et al. Enzymological analysis of mutant protein kinase C gamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem. 2008;283(28):19854–63.
Iwaki A et al. Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J Med Genet. 2008;45(1):32–5.
Marelli C et al. SCA15 due to large ITPR1 deletions in a cohort of 333 white families with dominant ataxia. Arch Neurol. 2011;68(5):637–43.
Di Gregorio E et al. Two Italian families with ITPR1 gene deletion presenting a broader phenotype of SCA15. Cerebellum. 2010;9(1):115–23.
Knight MA et al. Spinocerebellar ataxia type 15 (sca15) maps to 3p24.2-3pter: exclusion of the ITPR1 gene, the human orthologue of an ataxic mouse mutant. Neurobiol Dis. 2003;13(2):147–57.
Dougherty SE et al. Disruption of Purkinje cell function prior to huntingtin accumulation and cell loss in an animal model of Huntington disease. Exp Neurol. 2012;236(1):171–8.
Airaksinen MS et al. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci U S A. 1997;94(4):1488–93.
Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1(4):241–58.
Vecellio M et al. Alterations in Purkinje cell spines of calbindin D-28k and parvalbumin knock-out mice. Eur J Neurosci. 2000;12(3):945–54.
Serra HG et al. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet. 2004;13(20):2535–43.
Garthwaite G, Williams GD, Garthwaite J. Glutamate toxicity: an experimental and theoretical analysis. Eur J Neurosci. 1992;4(4):353–60.
Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286(5446):1882–8.
Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34(5):759–72.
Standley S et al. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28(3):887–98.
Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29(45):14371–80.
Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2(2):73–94.
Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Dev. 2009;12(5):693–704.
Lv RJ et al. ASIC1a polymorphism is associated with temporal lobe epilepsy. Epilepsy Res. 2011;96(1–2):74–80.
Liang L et al. The expression and phosphorylation of acid sensing ion channel 1a in the brain of a mouse model of phenylketonuria. Int J Neurosci. 2011;121(7):399–404.
Matricon J et al. Spinal cord plasticity and acid-sensing ion channels involvement in a rodent model of irritable bowel syndrome. Eur J Pain. 2011;15(4):335–43.
Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature. 2012;489(7416):400–5.
Acknowledgments
This work was partly supported by the University of Mississippi Medical Center’s Intramural Research Support Program Grant to PJS Vig.
Conflict of Interest
There is no conflict of interest. The data reported in this manuscript has not been published or submitted for publication elsewhere. All authors have agreed to the contents of this article and there are no ethical issues involved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vig, P.J.S., Hearst, S.M., Shao, Q. et al. Knockdown of Acid-Sensing Ion Channel 1a (ASIC1a) Suppresses Disease Phenotype in SCA1 Mouse Model. Cerebellum 13, 479–490 (2014). https://doi.org/10.1007/s12311-014-0563-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-014-0563-6