Abstract
Aggressive B-cell lymphomas, including the WHO diagnoses of diffuse large B-cell lymphoma, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, high-grade B-cell lymphoma, not otherwise specified, and Burkitt lymphoma, together account for approximately 40% of B-cell non-Hodgkin lymphomas. Identification of MYC, BCL2, and BCL6 rearrangements in these neoplasms is critical for diagnostic, prognostic, and therapeutic purposes. Herein, we address technical issues surrounding identification of these genetic abnormalities, discuss their diagnostic, prognostic, and therapeutic implications, and present an algorithm for use of interphase FISH in the work-up of aggressive B-cell lymphomas. To maximize sensitivity while still limiting cost, it is recommended that interphase FISH be performed in all B-cell lymphomas with large cell or high-grade morphology using both IGH/MYC dual-color dual-fusion (D-FISH) and MYC break-apart probes (BAP) as an initial probe set, followed by BCL2 BAP (or IGH/BCL2 D-FISH) and BCL6 BAP if a MYC rearrangement is identified. In pediatric patients or aggressive B-cell lymphomas with Burkitt-like morphology, a complete analysis at the outset using BAP probes for MYC, BCL2 (or IGH/BCL2 D-FISH), and BCL6 as well as D-FISH probes for IGH/MYC, IGK/MYC, and IGL/MYC is recommended.
Similar content being viewed by others
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma diagnosed in the United States and accounts for approximately 30–40% of all non-Hodgkin lymphomas [1]. Of cases morphologically classified as DLBCL, approximately 4–8% will be reclassified as the more aggressive entity designated “High-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements” per WHO 2017 criteria (DHL/THL, also referred to as “double-hit” or “triple-hit” lymphoma) [2, 3]. B-cell lymphomas with high-grade morphology, while less common overall, also frequently possess MYC and BCL2 and/or BCL6 rearrangements and are thus often reclassified as DHL/THL. The identification of an isolated MYC rearrangement (MYC-R) in B-cell lymphomas with large cell or high-grade morphology (“single hit lymphoma” SHL) may also confer a poorer prognosis, although this area is still controversial. Burkitt lymphoma nearly always possesses a MYC/immunoglobulin (IG) gene fusion, and although it typically has a uniform morphology and immunophenotype, its features may overlap with other aggressive B-cell lymphomas. As therapeutic regimens vary depending on the specific diagnosis, it is critical to establish the correct diagnosis for prognostic and therapeutic purposes.
Several methods have been used to detect gene rearrangements, including conventional cytogenetics, PCR, mate pair sequencing, and fluorescence in situ hybridization (FISH). However, conventional cytogenetics requires fresh tissue and may be hindered by the difficulty in obtaining suitable metaphases, PCR has low sensitivity, and mate-pair sequencing requires fresh or frozen tissue and at present is not widely available clinically. Interphase FISH, in contrast, has excellent sensitivity and specificity, is widely available, and it may be performed on formalin-fixed, paraffin-embedded (FFPE) material, including archival specimens and those with limited tissue [4,5,6]. Limitations of FISH often relate to specimen quality and processing prior to evaluation. For example, FISH testing performed on decalcified specimens, such as bone marrow trephine biopsies or bone-based lesions, may have limited success depending on the decalcifying agent used [7]. Additionally, approximately 20% of DLBCL cases have tumor necrosis at diagnosis [8], which may preclude accurate FISH testing on small biopsies.
Herein, we address the use of interphase FISH and propose an algorithm to aid in diagnosis of aggressive B-cell lymphomas, including the WHO diagnoses of DLBCL, DHL/THL, high-grade B-cell lymphoma, not otherwise specified (HGBL-NOS), and Burkitt lymphoma (BL). We also discuss the current challenges faced by pathologists and clinicians regarding interpretation of immunohistochemical (IHC) and cytogenetic findings in these cases. Beyond the scope of this paper are B-cell lymphomas such as plasmablastic lymphoma, blastoid mantle cell lymphoma, and others which may show MYC-R but remain within their respective diagnostic classifications. Also not discussed here are the gene expression profiles and/or mutational burden of these neoplasms. In this manuscript, concurrent MYC and BCL2 rearrangements, concurrent MYC and BCL6 rearrangements, and concurrent MYC, BCL2, and BCL6 rearrangements are referred to as MYC/BCL2, MYC/BCL6, and MYC/BCL2/BCL6, respectively.
Fish probe selection for the identification of MYC, BCL2, and BCL6 rearrangements
Several commercial FISH probe strategies used to test for MYC-R are available. The most commonly utilized FISH probes include IGH/MYC dual-color dual-fusion (D-FISH) and MYC break-apart probes (BAP). Due to the complex nature of rearrangements involving MYC at 8q24, false negatives may occur with either modality alone [9,10,11,12,13]. In one study, false negative rates (i.e., “normal” results, with no evidence of a MYC-R using a MYC BAP probe and no evidence of IGH/MYC fusion nor additional MYC signals compared to centromere 8 using an IGH/MYC D-FISH probe) were 4.1% and 22.1% using probes for MYC BAP and IGH/MYC D-FISH alone, respectively [12]. Furthermore, only about 45% of MYC-rearranged cases harbor IGH/MYC fusion, and thus, additional false negatives may occur in the setting of non-IGH partners. MYC BAP probes often fail to detect distal telomeric MYC translocation events, while IGH/MYC D-FISH probes may yield false negative results due to cryptic insertional mechanisms. There is also varying sensitivity among commercially available MYC BAP probes depending on extent of breakpoint region coverage, with some probes lacking distal 5′MYC or 3′MYC gene coverage [11]. Therefore, to maximize sensitivity and minimize turnaround time, it is recommended that both IGH/MYC D-FISH and MYC BAP probes be performed concurrently, with the caveat that cases with non-IGH/MYC fusions that are falsely negative by MYC BAP, will not be detected. Detection of these cases would require fresh/frozen tissue and sophisticated mate pair/whole genome sequencing methods that are not readily available for routine clinical use. An alternate approach in which MYC BAP is performed initially, with reflex to IGH/MYC D-FISH only if MYC BAP is negative, is also valid. The latter approach is less costly but would lengthen the turnaround time. In discordant cases, characterization of exact breakpoints may be determined by other genomic technologies such as next-generation sequencing.
To identify rearrangements of BCL2 and BCL6 by FISH, BAP probes rather than D-FISH probes are recommended. BCL6 is known to have numerous translocation partners, including immunoglobulin genes and multiple non-immunoglobulin genes [14]. BCL2 is almost always rearranged with IGH [15], although BCL2 rearrangements to IGK and IGL have been described [13, 16]. Advantages of using BCL2 BAP include ease of interpretation in FFPE as well as distinction of gains of gene regions on chromosome 18 from BCL2 rearrangements to non-IGH genes. However, false negative results using BCL2 BAP have been reported [13], although data remain limited. Therefore, for labs which offer only IGH/BCL2 D-FISH or prefer this method, IGH/BCL2 D-FISH remains an acceptable alternative to BAP.
MYC gene deregulation and tumorigenesis
The MYC proto-oncogene encodes the Myc protein, which is a nuclear transcription factor involved in multiple cellular functions including proliferation and apoptosis. Deregulation of MYC (e.g., via a translocation event) is a key factor in the pathogenesis of Burkitt lymphoma and many other lymphomas and malignancies [17]. The current understanding is that isolated MYC deregulation is not in itself tumorigenic as its increased apoptotic function keeps cellular proliferation in check. However, once MYC deregulation is coupled with another molecular event, such as TP53 mutations in Burkitt lymphoma, or BCL2/BCL6 rearrangements upregulating anti-apoptosis in DHL/THL, then tumorigenesis may occur [17, 18].
Prognostic implications of MYC rearrangements
High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements
DHL/THL was established as a diagnostic category in the 2017 revision of the World Health Organization (WHO) classification of lymphoid neoplasms because of its poor prognosis. These lymphomas are defined by the presence of MYC-R with an additional rearrangement in either BCL2, BCL6, or both and have a morphologic appearance that can vary from DLBCL-like (large cell) to intermediate between Burkitt lymphoma and DLBCL (high-grade) to blastoid [19]. DHL/THL often presents with advanced disease stage, and involvement of bone marrow and extranodal sites such as central nervous system is common. Median overall survival is less than 2 years, and DHL/THL has significantly poorer overall and progression-free survival than DLBCL [20,21,22,23,24,25,26]. However, some patients survive long term, with 5-year overall survival rates up to 49% [23].
Within the category of DHL/THL, the presence of high-grade cytologic features may confer a particularly poor survival [23, 27], and thus, the WHO recommends noting the morphologic appearance in all DHL/THL [19]. No significant survival differences between HGBL with MYC/BCL2 and HGBL with MYC/BCL2/BCL6 have been elucidated [24, 28, 29]. Some groups have observed improved survival in HGBL with MYC/BCL6 [23, 30, 31], but this remains controversial. Another contentious issue is the prognostic implications of MYC translocation partners. In DHL/THL, approximately 45% of MYC rearrangements involve IGH [t(8;14)(q24;q32)], and about 15% involve IGL (22q11.2) or IGK (2p11.2) [23]. In the remaining 40% of cases, MYC is translocated to a variety of non-immunoglobulin partners (non-IG-MYC), including, but not limited to, BCAS2, BCL6, BCL11A, IKZF1, IMMP2L, IRF4, and PAX5 [32, 33]. Some groups have demonstrated that DHL/THL with IG-MYC fusion have a poor prognosis, while those with non-IG-MYC fusion have a survival pattern similar to non-MYC-R DLBCL [24, 27, 30, 34]. However, other researchers have shown equally poor outcomes for DHL/THL patients regardless of IG-MYC versus non-IG-MYC fusion status [23, 35]. Resolution of this controversy is hampered by variation in cohort size and lack of uniformity regarding morphology, de novo versus transformed disease, and treatment regimens.
MYC-R in the absence of BCL2 and BCL6 rearrangements
The three morphologic variants of aggressive B cell lymphomas (Burkitt-like, high-grade, and large cell) may all harbor MYC-R in the absence of BCL2 and BCL6 gene rearrangements. Most cases with Burkitt-like morphology, characteristic immunophenotypic features, and IG-MYC fusion are diagnosed as Burkitt lymphoma, particularly in pediatric patients [19, 36]. However, as rare cases of pediatric DHL/THL have been described [37], and as DHL/THL may have Burkitt-like morphology, it is critical to evaluate for rearrangements involving MYC, BCL2, and BCL6 in every case of aggressive B-cell lymphoma in children and/or with Burkitt-like morphology to establish the correct diagnosis. Furthermore, pediatric patients with pathologic features suspicious for Burkitt lymphoma who lack MYC-R may carry a diagnosis of Burkitt-like lymphoma with 11q aberration, and chromosomal microarray analysis to investigate this possibility may be indicated [38]. In laboratories where chromosomal microarray is unavailable, FISH studies using probes specific to the regions of interest may have appropriate sensitivity; however, data are limited and additional confirmatory studies may be needed [39].
High-grade B-cell lymphoma, not otherwise specified (HGBL-NOS), per 2017 WHO criteria, is an aggressive B-cell lymphoma with high-grade morphology that lacks the cytogenetic features of DHL/THL, although it may have an isolated MYC-R (20–35% of cases) [40]. Aggressive B-cell lymphomas with large cell morphology may also have as isolated MYC-R (4% of cases) [3] and are still diagnosed as DLBCL, NOS. These two categories together have been colloquially referred to as “single hit lymphomas” (SHL). The prognostic significance of SHL is controversial. Data are limited regarding the prognostic significance of isolated MYC-R in HGBL-NOS due to small cohort sizes [19, 40, 41]. Regarding large cell morphology, a recent large retrospective study by Rosenwald et al. [24] showed that DLBCL patients with MYC-R have a statistically similar progression-free survival and overall survival to non-MYC-R DLBCL. This is in contrast to several other smaller cohorts showing that patients with SHL may have a poor prognosis similar to DHL/THL [30, 42,43,44,45,46].
MYC and BCL2 protein expression by immunohistochemistry in MYC-R
Not all large B cell lymphomas with MYC-R, including DHL/THLs, have poor survival [22], and several groups have tried to identify factors that are associated with better survival. Only about 80% of DHL/THL express both MYC and BCL2 protein (“double-expressers”; DEL), even though the vast majority possess both MYC and BCL2 rearrangements. The remaining 20% of DHL/THL may have a more favorable outcome, although data are limited [27, 47, 48]. MYC-R may only confer a worse prognosis if MYC expression is also increased [45, 46], and conversely, elevated MYC protein expression may confer a poor prognosis regardless of MYC-R status [49,50,51,52]. Others have found that MYC-R is only significant if also accompanied by BCL2 protein overexpression [47, 53]. It has been proposed that in cases both with and without MYC-R, the degree of MYC and BCL2 protein expression is proportional to clinical risk [54].
Treatment implications of MYC-R
Historically, patients with MYC-rearranged aggressive B cell lymphomas do poorly with standard dose R-CHOP chemotherapy or R-CHOP-like regimens. Patients who can tolerate it may benefit from more intensive induction regimens [22, 55]. More aggressive induction regimens are typically used in patients with DHL/THL [22, 23, 56], although this remains an area of clinical uncertainty. While data from single arm studies and observational studies suggests that patients with MYC-R DLBCL and DHL/THL that receive more aggressive regimens such as dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R) may trend towards similar overall survival to those with non-MYC-R DLBCL [23, 57], there are no definite randomized studies proving that intensified chemotherapy is indeed superior to standard R-CHOP used in DLBCL. Newer targeted therapies, such as the anti-BCL2 venetoclax, may prove to be an effective option [55, 58], and a randomized study sponsored by the National Cancer Institute is ongoing (NCT03984448). As yet, targeted therapies which directly inhibit MYC are elusive [59, 60]. Autologous stem cell transplants have been attempted in patients with relapsed or refractory DHL/THL with poor results, but remain an option [48]. Early results of chimeric antigen T cell receptor (CART) therapy have shown promising results in relapse/refectory setting [61] and will likely lead to development of post induction chemotherapy consolidative approaches in high-risk patients.
When to perform FISH for MYC, BCL2, and BCL6 rearrangements
Recommended: perform FISH on every aggressive B cell lymphoma
Because of the prognostic and therapeutic implications of DHL/THL, all new aggressive B cell lymphoma diagnoses should be accompanied by appropriate FISH testing to rule out DHL/THL when biopsy tissue quantity and quality permit. One important argument for testing all DLBCL specimens is that DHL/THL with DLBCL morphology is uniquely defined by genetics. Considering that DLBCL is the most common lymphoma in the United States, cost and turnaround time issues will understandably arise in certain practices which lack the resources and capability of performing these assays. For those that cannot manage this approach, or when small specimen size precludes full workup, selective FISH strategies are also outlined below.
Proposed algorithms for the order of FISH testing
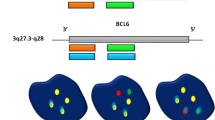
An algorithmic approach for performing interphase FISH testing in B cell lymphomas with large cell or high-grade morphology is presented in Fig. 1. Since all DHL/THL possess a rearrangement of MYC by definition and isolated MYC-R (SHL) may have prognostic significance, all cases are tested for a MYC rearrangement, using both BAP and IGH/MYC D-FISH probes to minimize false negative results; both are performed simultaneously to minimize turnaround time. This is followed by testing for both BCL2 and BCL6 translocations if MYC is rearranged. A similar algorithmic approach is recommended as essential diagnostic testing for DLBCL per the NCCN Guidelines Version 1.2020 [62]. However, depending on clinical practice, resources, and/or laboratory workflow, some practices may choose to test for rearrangements of MYC, BCL2, and BCL6 at once to expedite diagnosis of DHL/THL, although this will often result in unnecessary testing for BCL2 and BCL6 rearrangements.
In cases with classic Burkitt morphology or in pediatric populations where Burkitt lymphoma remains high in the differential diagnosis even without classic morphology, a modified algorithmic approach is proposed (Fig. 2). In these cases, testing using BAP probes for MYC, BCL2, and BCL6, as well as D-FISH probes for IGH/MYC, IGL/MYC, and IGK/MYC, is performed simultaneously. This expedites the analysis, which is especially important in Burkitt lymphoma given its highly aggressive biology. It also evaluates for all IG/MYC combinations, as non-IG/MYC rearrangements are extremely rare in Burkitt lymphoma. Finally, it excludes the unlikely possibility of DHL/THL [19].
Alternative: selective FISH strategies to predict MYC-R
Several studies have investigated the value of cell of origin (COO) testing to predict for DHL/THL, relying on the fact that approximately 85% of DHL/THL possess a BCL2 rearrangement and essentially all DHL/THL with BCL2 rearrangements are of germinal center B cell (GCB) phenotype [3, 23]. Furthermore, DHL/THL with BCL2 rearrangements have been most firmly established to have an aggressive clinical course and thus could potentially benefit most from more aggressive therapy. Since approximately 50% of DLBCL are of GCB phenotype [3], a laboratory could potentially cut the number of cases requiring FISH testing in half by using an IHC algorithm with appropriate specificity and sensitivity, such as the Hans or Choi algorithm, or a digital gene expression profiling assay such as Lymph2Cx to identify cases of GCB phenotype; however, each of these algorithms shows varying degrees of specificity, sensitivity, and reproducibility [63,64,65,66]. Performing FISH on only this cohort would detect 99% of DHL/THL with MYC/BCL2 or MYC/BCL2/BCL6. However, this strategy would miss about half of DHL/THL with MYC/BCL6 and about half of SHL, or about 25% of MYC-R cases overall [3].
It has also been proposed that coexpression of MYC and BCL2 proteins by IHC could be used to screen for DHL/THL, relying on the concept that gene rearrangements should result in increased expression of the corresponding protein in most cases. However, there are multiple problems with this approach. Firstly, assessing the percentage of tumor cells that express nuclear MYC protein by IHC has proven to be poorly reproducible among pathologists, particularly when MYC expression is near the 40% cutoff for positivity [67, 68]. MYC protein expression can vary throughout the tumor, shows significant variability in intensity of expression, and is affected by specific lab protocols and tissue fixation. Secondly, MYC protein expression status is a poor predictor of MYC-R, as there is no identified threshold for MYC IHC positivity that will predict MYC-R [3]. For example, using an IHC threshold of ≥ 40%, which was initially established for prognostic purposes and not to screen for MYC rearrangements, only 40% of DLBCLs that express MYC show MYC-R [3, 69]. Conversely, MYC protein is expressed in only about 80% of MYC-R cases. Scott et al. [3] outlined the percentage of DHL/THL cases that would be missed in aggressive B-cell lymphomas with large cell morphology if FISH testing was performed selectively depending on DEL ± COO status. If only DEL were investigated, one would expect to miss approximately 25% of DHL/THL, but would reduce testing to about 34% of DLBCL. If FISH investigation were further limited to DELs of GCB phenotype, FISH testing would be reduced to about 15% of all cases without any further decrease in sensitivity. In light of the poor sensitivity coupled with the difficulty in interpreting MYC expression by IHC reliably, this approach is not recommended.
Other factors such as age, IPI score, or disease stage are not good predictors of MYC rearrangement status [70]. Although most DHL/THL arise in older individuals, FISH testing for MYC, BCL2, and BCL6 gene rearrangements must be performed on all aggressive B-cell lymphomas arising in pediatric patients to exclude both Burkitt lymphoma and DHL/THL. Histologic appearance is not a good predictor of MYC-R status, as only a subset of aggressive BCL with high-grade features (33–58%) [40, 50] or large cell features (12%) [3] will possess a MYC-R. Ki-67 proliferation index as estimated by IHC is also a poor predictor of MYC-R status [47, 70]. Lack of LMO2 protein expression by IHC may predict for MYC-R in CD10-positive DLBCL [71], but additional studies are needed to verify this finding.
Conclusions
Identification of MYC, BCL2, and BCL6 translocations in aggressive B-cell lymphomas, in conjunction with clinical, morphologic, and immunophenotypic features, is important for diagnostic, prognostic, and therapeutic purposes. Because most DHL/THL and some SHL have poor prognoses and may warrant more aggressive treatment, identification of MYC rearrangements ± BCL2 and BCL6 rearrangements is critical. To maximize sensitivity while still limiting cost, it is recommended that interphase FISH be performed in all B-cell lymphomas with large cell or high-grade morphology using both IGH/MYC D-FISH and MYC BAP probes as an initial probe set, followed by BCL2 BAP (or IGH/BCL2 D-FISH) and BCL6 BAP probes if a MYC rearrangement is identified. In pediatric patients or aggressive B-cell lymphomas with Burkitt-like morphology, a complete analysis at the outset using BAP probes for MYC, BCL2 (or IGH/BCL2 D-FISH), and BCL6 as well as D-FISH probes for IGH/MYC, IGK/MYC, and IGL/MYC is recommended.
References
Menon MP, Pittaluga S, Jaffe ES (2012) The histological and biological spectrum of diffuse large B-cell lymphoma in the World Health Organization classification. Cancer J 18(5):411–420. https://doi.org/10.1097/PPO.0b013e31826aee97
Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, Espinosa I, Novelli S, Briones J, Mate JL, Salamero O, Sancho JM, Arenillas L, Serrano S, Erill N, Martinez D, Castillo P, Rovira J, Martinez A, Campo E, Colomo L, Grup per l'Estudi dels Limfomes de Catalunya i B (2013) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica 98(10):1554–1562. https://doi.org/10.3324/haematol.2013.086173
Scott DW, King RL, Staiger AM, Ben-Neriah S, Jiang A, Horn H, Mottok A, Farinha P, Slack GW, Ennishi D, Schmitz N, Pfreundschuh M, Nowakowski GS, Kahl BS, Connors JM, Gascoyne RD, Ott G, Macon WR, Rosenwald A (2018) High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 131(18):2060–2064. https://doi.org/10.1182/blood-2017-12-820605
Paternoster SF, Brockman SR, McClure RF, Remstein ED, Kurtin PJ, Dewald GW (2002) A new method to extract nuclei from paraffin-embedded tissue to study lymphomas using interphase fluorescence in situ hybridization. Am J Pathol 160(6):1967–1972. https://doi.org/10.1016/s0002-9440(10)61146-7
Ventura RA, Martin-Subero JI, Jones M, McParland J, Gesk S, Mason DY, Siebert R (2006) FISH analysis for the detection of lymphoma-associated chromosomal abnormalities in routine paraffin-embedded tissue. J Mol Diagn 8(2):141–151. https://doi.org/10.2353/jmoldx.2006.050083
Einerson RR, Kurtin PJ, Dayharsh GA, Kimlinger TK, Remstein ED (2005) FISH is superior to PCR in detecting t(14;18)(q32;q21)–IgH/bcl-2 in follicular lymphoma using paraffin-embedded tissue samples. Am J Clin Pathol 124(3):421–429. https://doi.org/10.1309/blh8mmk85ubq4k6r
Neat MJ, Moonim MT, Dunn RG, Geoghegan H, Foot NJ (2013) Fluorescence in situ hybridisation analysis of bone marrow trephine biopsy specimens; an additional tool in the diagnostic armoury. J Clin Pathol 66(1):54–57. https://doi.org/10.1136/jclinpath-2012-201131
Song MK, Chung JS, Shin DY, Lim SN, Lee GW, Choi JC, Park WY, Oh SY (2017) Tumor necrosis could reflect advanced disease status in patients with diffuse large B cell lymphoma treated with R-CHOP therapy. Ann Hematol 96(1):17–23. https://doi.org/10.1007/s00277-016-2822-8
Peterson JF, Pitel BA, Smoley SA, Vasmatzis G, Smadbeck JB, Greipp PT, Ketterling RP, Macon WR, Baughn LB (2019) Elucidating a false-negative MYC break-apart fluorescence in situ hybridization probe study by next-generation sequencing in a patient with high-grade B-cell lymphoma with IGH/MYC and IGH/BCL2 rearrangements. Cold Spring Harb Mol Case Stud 5(3):a004077. https://doi.org/10.1101/mcs.a004077
Wagener R, Bens S, Toprak UH, Seufert J, Lopez C, Scholz I, Herbrueggen H, Oschlies I, Stilgenbauer S, Schlesner M, Klapper W, Burkhardt B, Siebert R (2019) Cryptic insertion of MYC exons 2 and 3 into the IGH locus detected by whole genome sequencing in a case of MYC-negative Burkitt lymphoma. Haematologica. 105:e202–e205. https://doi.org/10.3324/haematol.2018.208140
Muñoz-Mármol AM, Sanz C, Tapia G, Marginet R, Ariza A, Mate JL (2013) MYC status determination in aggressive B-cell lymphoma: the impact of FISH probe selection. Histopathology 63(3):418–424. https://doi.org/10.1111/his.12178
King RL, McPhail ED, Meyer RG, Vasmatzis G, Pearce K, Smadbeck JB, Ketterling RP, Smoley SA, Greipp PT, Hoppman NL, Peterson JF, Baughn LB (2019) False-negative rates for MYC fluorescence in situ hybridization probes in B-cell neoplasms. Haematologica 104(6):e248–e251. https://doi.org/10.3324/haematol.2018.207290
Hilton LK, Tang J, Ben-Neriah S, Alcaide M, Jiang A, Grande BM, Rushton CK, Boyle M, Meissner B, Scott DW, Morin RD (2019) The double-hit signature identifies double-hit diffuse large B-cell lymphoma with genetic events cryptic to FISH. Blood 134(18):1528–1532. https://doi.org/10.1182/blood.2019002600
Ohno H (2006) Pathogenetic and clinical implications of non-immunoglobulin; BCL6 translocations in B-cell non-Hodgkin's lymphoma. J Clin Exp Hematop 46(2):43–53. https://doi.org/10.3960/jslrt.46.43
Kramer MHH (1998) Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood 92(9):3152–3162. https://doi.org/10.1182/blood.V92.9.3152
Bentley G, Palutke M, Mohamed AN (2005) Variant t(14;18) in malignant lymphoma: a report of seven cases. Cancer Genet Cytogenet 157(1):12–17. https://doi.org/10.1016/j.cancergencyto.2004.05.012
Ott G (2014) Impact of MYC on malignant behavior. Hematology Am Soc Hematol Educ Program 2014(1):100–106. https://doi.org/10.1182/asheducation-2014.1.100
Korać P, Dotlić S, Matulić M, Zajc Petranović M, Dominis M (2017) Role of MYC in B cell Lymphomagenesis. Genes 8(4):115. https://doi.org/10.3390/genes8040115
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri S, Stein H, Thiele JE (2017) WHO classification of tumours of haematopoietic and lymphoid tissues, Revised 4th edn. IARC, Lyon
McClure RE, Remstein ED, Macon WR, Dewald GW, Habermann TM, Hoering A, Kurtin PJ (2005) Adult B-cell lymphomas with Burkitt-like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol 29(12):1652–1660. https://doi.org/10.1097/01.pas.0000180442.87022.08
Le Gouill S, Talmant P, Touzeau C, Moreau A, Garand R, Juge-Morineau N, Gaillard F, Gastinne T, Milpied N, Moreau P, Harousseau JL, Avet-Loiseau H (2007) The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica 92(10):1335–1342. https://doi.org/10.3324/haematol.11305
Petrich AM, Gandhi M, Jovanovic B, Castillo JJ, Rajguru S, Yang DT, Shah KA, Whyman JD, Lansigan F, Hernandez-Ilizaliturri FJ, Lee LX, Barta SK, Melinamani S, Karmali R, Adeimy C, Smith S, Dalal N, Nabhan C, Peace D, Vose J, Evens AM, Shah N, Fenske TS, Zelenetz AD, Landsburg DJ, Howlett C, Mato A, Jaglal M, Chavez JC, Tsai JP, Reddy N, Li S, Handler C, Flowers CR, Cohen JB, Blum KA, Song K, Sun HL, Press O, Cassaday R, Jaso J, Medeiros LJ, Sohani AR, Abramson JS (2014) Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 124(15):2354–2361. https://doi.org/10.1182/blood-2014-05-578963
McPhail ED, Maurer MJ, Macon WR, Feldman AL, Kurtin PJ, Ketterling RP, Vaidya R, Cerhan JR, Ansell SM, Porrata LF, Nowakowski GS, Witzig TE, Habermann TM (2018) Inferior survival in high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements is not associated with MYC/IG gene rearrangements. Haematologica 103(11):1899–1907. https://doi.org/10.3324/haematol.2018.190157
Rosenwald A, Bens S, Advani R, Barrans S, Copie-Bergman C, Elsensohn M-H, Natkunam Y, Calaminici M, Sander B, Baia M, Smith A, Painter D, Pham L, Zhao S, Ziepert M, Jordanova ES, Molina TJ, Kersten MJ, Kimby E, Klapper W, Raemaekers J, Schmitz N, Jardin F, Stevens WBC, Hoster E, Hagenbeek A, Gribben JG, Siebert R, Gascoyne RD, Scott DW, Gaulard P, Salles G, Burton C, De Jong D, Sehn LH, Maucort-Boulch D (2019) Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the Lunenburg lymphoma biomarker consortium. J Clin Oncol 37:3359–3368. https://doi.org/10.1200/jco.19.00743
Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A (2010) Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 28(20):3360–3365. https://doi.org/10.1200/jco.2009.26.3947
Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, Loeffler M (2010) Standard international prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 28(14):2373–2380. https://doi.org/10.1200/JCO.2009.26.2493
Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, Dyer MJ, Siebert R, Kuruvilla J, Klasa R, Connors JM, Gascoyne RD, Horsman DE (2009) Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 114(11):2273–2279. https://doi.org/10.1182/blood-2009-03-212191
Li S, Desai P, Lin P, Yin CC, Tang G, Wang XJ, Konoplev SN, Khoury JD, Bueso-Ramos CE, Medeiros LJ (2016) MYC/BCL6 double-hit lymphoma (DHL): a tumour associated with an aggressive clinical course and poor prognosis. Histopathology 68(7):1090–1098. https://doi.org/10.1111/his.12884
Huang W, Medeiros LJ, Lin P, Wang W, Tang G, Khoury J, Konoplev S, Yin CC, Xu J, Oki Y, Li S (2018) MYC/BCL2/BCL6 triple hit lymphoma: a study of 40 patients with a comparison to MYC/BCL2 and MYC/BCL6 double hit lymphomas. Mod Pathol 31(9):1470–1478. https://doi.org/10.1038/s41379-018-0067-x
Copie-Bergman C, Cuilliere-Dartigues P, Baia M, Briere J, Delarue R, Canioni D, Salles G, Parrens M, Belhadj K, Fabiani B, Recher C, Petrella T, Ketterer N, Peyrade F, Haioun C, Nagel I, Siebert R, Jardin F, Leroy K, Jais JP, Tilly H, Molina TJ, Gaulard P (2015) MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood 126(22):2466–2474. https://doi.org/10.1182/blood-2015-05-647602
Clipson A, Barrans S, Zeng N, Crouch S, Grigoropoulos NF, Liu H, Kocialkowski S, Wang M, Huang Y, Worrillow L, Goodlad J, Buxton J, Neat M, Fields P, Wilkins B, Grant JW, Wright P, Ei-Daly H, Follows GA, Roman E, Watkins AJ, Johnson PW, Jack A, Du MQ (2015) The prognosis of MYC translocation positive diffuse large B-cell lymphoma depends on the second hit. J Pathol Clin Res 1(3):125–133. https://doi.org/10.1002/cjp2.10
Bertrand P, Bastard C, Maingonnat C, Jardin F, Maisonneuve C, Courel MN, Ruminy P, Picquenot JM, Tilly H (2007) Mapping of MYC breakpoints in 8q24 rearrangements involving non-immunoglobulin partners in B-cell lymphomas. Leukemia 21(3):515–523. https://doi.org/10.1038/sj.leu.2404529
Otto C, Scholtysik R, Schmitz R, Kreuz M, Becher C, Hummel M, Rosenwald A, Trümper L, Klapper W, Siebert R, Küppers R (2016) Novel IGH and MYC translocation partners in diffuse large B-cell lymphomas. Genes Chromosom Cancer 55(12):932–943. https://doi.org/10.1002/gcc.22391
Pedersen MØ, Gang AO, Poulsen TS, Knudsen H, Lauritzen AF, Nielsen SL, Klausen TW, Nørgaard P (2014) MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol 92(1):42–48. https://doi.org/10.1111/ejh.12212
Aukema SM, Kreuz M, Kohler CW, Rosolowski M, Hasenclever D, Hummel M, Kuppers R, Lenze D, Ott G, Pott C, Richter J, Rosenwald A, Szczepanowski M, Schwaenen C, Stein H, Trautmann H, Wessendorf S, Trumper L, Loeffler M, Spang R, Kluin PM, Klapper W, Siebert R, Molecular Mechanisms in Malignant Lymphomas Network P (2014) Biological characterization of adult MYC-translocation-positive mature B-cell lymphomas other than molecular Burkitt lymphoma. Haematologica 99(4):726–735. https://doi.org/10.3324/haematol.2013.091827
Salaverria I, Siebert R (2011) The gray zone between Burkitt's lymphoma and diffuse large B-cell lymphoma from a genetics perspective. J Clin Oncol 29(14):1835–1843. https://doi.org/10.1200/jco.2010.32.8385
Gualco G, Weiss LM, Harrington WJ, Bacchi CE (2009) Nodal diffuse large B-cell lymphomas in children and adolescents: immunohistochemical expression patterns and c-MYC translocation in relation to clinical outcome. Am J Surg Pathol 33(12):1815–1822. https://doi.org/10.1097/pas.0b013e3181bb9a18
Salaverria I, Martin-Guerrero I, Wagener R, Kreuz M, Kohler CW, Richter J, Pienkowska-Grela B, Adam P, Burkhardt B, Claviez A, Damm-Welk C, Drexler HG, Hummel M, Jaffe ES, Kuppers R, Lefebvre C, Lisfeld J, Loffler M, Macleod RAF, Nagel I, Oschlies I, Rosolowski M, Russell RB, Rymkiewicz G, Schindler D, Schlesner M, Scholtysik R, Schwaenen C, Spang R, Szczepanowski M, Trumper L, Vater I, Wessendorf S, Klapper W, Siebert R (2014) A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood 123(8):1187–1198. https://doi.org/10.1182/blood-2013-06-507996
Gonzalez-Farre B, Ramis-Zaldivar JE, Salmeron-Villalobos J, Balagué O, Celis V, Verdu-Amoros J, Nadeu F, Sábado C, Ferrández A, Garrido M, García-Bragado F, De La Maya MD, Vagace JM, Panizo CM, Astigarraga I, Andrés M, Jaffe ES, Campo E, Salaverria I (2019) Burkitt-like lymphoma with 11q aberration: a germinal center derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica 104(9):1822–1829. https://doi.org/10.3324/haematol.2018.207928
Lin P, Dickason TJ, Fayad LE, Lennon PA, Hu P, Garcia M, Routbort MJ, Miranda R, Wang X, Qiao W, Medeiros LJ (2012) Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer 118(6):1566–1573. https://doi.org/10.1002/cncr.26433
Perry AM, Crockett D, Dave BJ, Althof P, Winkler L, Smith LM, Aoun P, Chan WC, Fu K, Greiner TC, Bierman P, Gregory Bociek R, Vose JM, Armitage JO, Weisenburger DD (2013) B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and burkitt lymphoma: study of 39 cases. Brit J Haematol 162(1):40–49. https://doi.org/10.1111/bjh.12343
Wang XJ, Reddy N, Li S (2013) MYC gene single hit diffuse large B cell lymphoma behaves similarly to MYC/BCL2 double hit lymphoma and needs similar aggressive treatment. Blood 122(21):4313. https://doi.org/10.1182/blood.V122.21.4313.4313
Landsburg DJ, Falkiewicz MK, Petrich AM, Chu BA, Behdad A, Li SY, Medeiros LJ, Cassaday RD, Reddy NM, Bast MA, Vose JM, Kruczek KR, Smith SE, Patel P, Hernandez-Ilizaliturri F, Karmali R, Rajguru S, Yang DT, Maly JJ, Blum KA, Zhao WQ, Vanslambrouck C, Nabhan C (2016) Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Brit J Haematol 175(4):631–640. https://doi.org/10.1111/bjh.14282
Li SY, Weiss VL, Wang XJ, Desai PA, Hu SM, Yin CC, Tang GL, Reddy NM, Medeiros LJ, Lin P (2016) High-grade B-cell lymphoma with MYC rearrangement and without BCL2 and BCL6 rearrangements is associated with high P53 expression and a poor prognosis. Am J Surg Pathol 40(2):253–261. https://doi.org/10.1097/Pas.0000000000000542
Pedersen MØ, Gang AO, Clasen-Linde E, Breinholt MF, Knudsen H, Nielsen SL, Poulsen TS, Klausen TW, Høgdall E, Nørgaard P (2019) Stratification by MYC expression has prognostic impact in MYC translocated B-cell lymphoma-identifies a subgroup of patients with poor outcome. Eur J Haematol 102(5):395–406. https://doi.org/10.1111/ejh.13219
Tzankov A, Xu-Monette ZY, Gerhard M, Visco C, Dirnhofer S, Gisin N, Dybkaer K, Orazi A, Bhagat G, Richards KL, Hsi ED, Choi WW, van Krieken JH, Ponzoni M, Ferreri AJ, Ye Q, Winter JN, Farnen JP, Piris MA, Moller MB, You MJ, McDonnell T, Medeiros LJ, Young KH (2014) Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol 27(7):958–971. https://doi.org/10.1038/modpathol.2013.214
Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, Chan WC, Iqbal J, Meyer PN, Lenz G, Wright G, Rimsza LM, Valentino C, Brunhoeber P, Grogan TM, Braziel RM, Cook JR, Tubbs RR, Weisenburger DD, Campo E, Rosenwald A, Ott G, Delabie J, Holcroft C, Jaffe ES, Staudt LM, Gascoyne RD (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30(28):3452–3459. https://doi.org/10.1200/JCO.2011.41.0985
Herrera AF, Mei M, Low L, Kim HT, Griffin GK, Song JY, Merryman RW, Bedell V, Pak C, Sun H, Paris T, Stiller T, Brown JR, Budde LE, Chan WC, Chen R, Davids MS, Freedman AS, Fisher DC, Jacobsen ED, Jacobson CA, LaCasce AS, Murata-Collins J, Nademanee AP, Palmer JM, Pihan GA, Pillai R, Popplewell L, Siddiqi T, Sohani AR, Zain J, Rosen ST, Kwak LW, Weinstock DM, Forman SJ, Weisenburger DD, Kim Y, Rodig SJ, Krishnan A, Armand P (2017) Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J Clin Oncol 35(1):24–31. https://doi.org/10.1200/JCO.2016.68.2740
Kluk MJ, Chapuy B, Sinha P, Roy A, Cin PD, Neuberg DS, Monti S, Pinkus GS, Shipp MA, Rodig SJ (2012) Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One 7(4):e33813. https://doi.org/10.1371/journal.pone.0033813
Cook JR, Goldman B, Tubbs RR, Rimsza L, Leblanc M, Stiff P, Fisher R (2014) Clinical significance of MYC expression and/or "high-grade" morphology in non-Burkitt, diffuse aggressive B-cell lymphomas: a SWOG S9704 correlative study. Am J Surg Pathol 38(4):494–501. https://doi.org/10.1097/PAS.0000000000000147
Xu-Monette ZY, Dabaja BS, Wang X, Tu M, Manyam GC, Tzankov A, Xia Y, Zhang L, Sun R, Visco C, Dybkaer K, Yin L, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, van Krieken JH, Huh J, Ponzoni M, Ferreri AJ, Moller MB, Parsons BM, Zhao X, Winter JN, Piris MA, McDonnell TJ, Miranda RN, Li Y, Medeiros LJ, Young KH (2015) Clinical features, tumor biology, and prognosis associated with MYC rearrangement and Myc overexpression in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol 28(12):1555–1573. https://doi.org/10.1038/modpathol.2015.118
Ziepert M, Lazzi S, Santi R, Vergoni F, Granai M, Mancini V, Staiger A, Horn H, Loffler M, Poschel V, Held G, Wulf G, Trumper LH, Schmitz N, Rosenwald A, Sabattini E, Naresh KN, Stein H, Ott G, Leoncini L (2020) A 70% cut-off for MYC protein expression in diffuse large B cell lymphoma identifies a high-risk group of patients. Haematologica. https://doi.org/10.3324/haematol.2019.235556
Kawamoto K, Miyoshi H, Yoshida N, Nakamura N, Ohshima K, Sone H, Takizawa J (2016) MYC translocation and/or BCL2 protein expression are associated with poor prognosis in diffuse large B-cell lymphoma. Cancer Sci 107(6):853–861. https://doi.org/10.1111/cas.12942
Sesques P, Johnson NA (2017) Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood 129(3):280–288. https://doi.org/10.1182/blood-2016-02-636316
de Jonge AV, Roosma TJ, Houtenbos I, Vasmel WL, van de Hem K, de Boer JP, van Maanen T, Lindauer-van der Werf G, Beeker A, Timmers GJ, Schaar CG, Soesan M, Poddighe PJ, de Jong D, Chamuleau ME (2016) Diffuse large B-cell lymphoma with MYC gene rearrangements: current perspective on treatment of diffuse large B-cell lymphoma with MYC gene rearrangements; case series and review of the literature. Eur J Cancer 55:140–146. https://doi.org/10.1016/j.ejca.2015.12.001
Sun H, Savage KJ, Karsan A, Slack GW, Gascoyne RD, Toze CL, Sehn LH, Abou Mourad Y, Barnett MJ, Broady RC, Connors JM, Forrest DL, Gerrie AS, Hogge DE, Narayanan S, Nevill TJ, Nantel SH, Power MM, Sutherland HJ, Villa D, Shepherd JD, Song KW (2015) Outcome of patients with non-Hodgkin lymphomas with concurrent MYC and BCL2 rearrangements treated with CODOX-M/IVAC with rituximab followed by hematopoietic stem cell transplantation. Clin Lymphoma Myeloma Leuk 15(6):341–348. https://doi.org/10.1016/j.clml.2014.12.015
Lai C, Roschewski M, Melani C, Pittaluga S, Shovlin M, Steinberg SM, Dunleavy K, Pack S, Jaffe ES, Wilson WH (2018) MYC gene rearrangement in diffuse large B-cell lymphoma does not confer a worse prognosis following dose-adjusted EPOCH-R. Leuk Lymphoma 59(2):505–508. https://doi.org/10.1080/10428194.2017.1339882
Uchida A, Isobe Y, Asano J, Uemura Y, Hoshikawa M, Takagi M, Miura I (2019) Targeting BCL2 with venetoclax is a promising therapeutic strategy for "double-proteinexpression" lymphoma with MYC and BCL2 rearrangements. Haematologica 104(7):1417–1421. https://doi.org/10.3324/haematol.2018.204958
McKeown MR, Bradner JE (2014) Therapeutic strategies to inhibit MYC. Cold Spring Harb Perspect Med 4(10). https://doi.org/10.1101/cshperspect.a014266
Koh CM, Sabo A, Guccione E (2016) Targeting MYC in cancer therapy: RNA processing offers new opportunities. Bioessays 38(3):266–275. https://doi.org/10.1002/bies.201500134
Abramson JS, Siddiqi T, Palomba ML, Gordon LI, Lunning MA, Arnason JE, Wang M, Forero-Torres A, Albertson T, Dehner C, Garcia J, Li D, Xie B, Maloney DG (2018) High durable CR rates and preliminary safety profile for JCAR017 in R/R aggressive b-NHL (TRANSCEND NHL 001 study): a defined composition CD19-directed CAR T-cell product with potential for outpatient administration. J Clin Oncol 36(5_suppl):120–120. https://doi.org/10.1200/JCO.2018.36.5_suppl.120
National Comprehensive Cancer Network (2020) Diffuse large B-cell lymphoma (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed 5/20/2020
Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, Ott G, Rosenwald A, Braziel RM, Campo E, Vose JM, Lenz G, Staudt LM, Chan WC, Weisenburger DD (2011) Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol 29(2):200–207. https://doi.org/10.1200/jco.2010.30.0368
Culpin RE, Sieniawski M, Angus B, Menon GK, Proctor SJ, Milne P, McCabe K, Mainou-Fowler T (2013) Prognostic significance of immunohistochemistry-based markers and algorithms in immunochemotherapy-treated diffuse large B cell lymphoma patients. Histopathology 63(6):788–801. https://doi.org/10.1111/his.12223
Hwang HS, Park C-S, Yoon DH, Suh C, Huh J (2014) High concordance of gene expression profiling–correlated immunohistochemistry algorithms in diffuse large B-cell lymphoma, not otherwise specified. Am J Surg Pathol 38(8):1046–1057. https://doi.org/10.1097/pas.0000000000000211
Scott DW, Mottok A, Ennishi D, Wright GW, Farinha P, Ben-Neriah S, Kridel R, Barry GS, Hother C, Abrisqueta P, Boyle M, Meissner B, Telenius A, Savage KJ, Sehn LH, Slack GW, Steidl C, Staudt LM, Connors JM, Rimsza LM, Gascoyne RD (2015) Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol 33(26):2848–2856. https://doi.org/10.1200/jco.2014.60.2383
Ambrosio MR, Lazzi S, Bello GL, Santi R, Porro LD, de Santi MM, Guazzo R, Mundo L, Rigacci L, Kovalchuck S, Onyango N, Fabbri A, Cencini E, Zinzani PL, Zaja F, Angrilli F, Stelitano C, Cabras MG, Spataro G, Bob R, Menter T, Granai M, Cevenini G, Naresh KN, Stein H, Sabattini E, Leoncini L (2019) MYC protein expression scoring and its impact on the prognosis of aggressive B-cell lymphoma patients. Haematologica 104(1):e25–e28. https://doi.org/10.3324/haematol.2018.195958
Mahmoud AZ, George TI, Czuchlewski DR, Zhang Q-Y, Wilson CS, Sever CE, Bakhirev AG, Zhang D, Steidler NL, Reichard KK, Kang H, Foucar K, Vasef MA (2015) Scoring of MYC protein expression in diffuse large B-cell lymphomas: concordance rate among hematopathologists. Mod Pathol 28(4):545–551. https://doi.org/10.1038/modpathol.2014.140
Raess PW, Moore SR, Cascio MJ, Dunlap J, Fan G, Gatter K, Olson SB, Braziel RM (2018) MYC immunohistochemical and cytogenetic analysis are required for identification of clinically relevant aggressive B cell lymphoma subtypes. Leuk Lymphoma 59(6):1391–1398. https://doi.org/10.1080/10428194.2017.1370547
Landsburg DJ, Nasta SD, Svoboda J, Morrissette JJD, Schuster SJ (2014) ‘Double-hit’ cytogenetic status may not be predicted by baseline clinicopathological characteristics and is highly associated with overall survival in B cell lymphoma patients. Brit J Haematol 166(3):369–374. https://doi.org/10.1111/bjh.12901
Colomo L, Vazquez I, Papaleo N, Espinet B, Ferrer A, Franco C, Comerma L, Hernandez S, Calvo X, Salar A, Climent F, Mate JL, Forcada P, Mozos A, Nonell L, Martinez A, Carrio A, Costa D, Dlouhy I, Salaverria I, Martin-Subero JI, Lopez-Guillermo A, Valera A, Campo E, Grup per l'Estudi dels Limfomes de Catalunya i B (2017) LMO2-negative expression predicts the presence of MYC translocations in aggressive B-cell lymphomas. Am J Surg Pathol 41(7):877–886. https://doi.org/10.1097/PAS.0000000000000839
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Larson, D.P., Peterson, J.F., Nowakowski, G.S. et al. A practical approach to FISH testing for MYC rearrangements and brief review of MYC in aggressive B-cell lymphomas. J Hematopathol 13, 127–135 (2020). https://doi.org/10.1007/s12308-020-00404-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-020-00404-w