Abstract
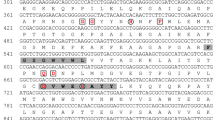
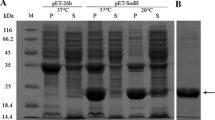
Increasing evidence indicates that the biosynthesis and accumulation of the keto-carotenoid astaxanthin represents a chronic (in days or weeks) molecular defense mechanism in the green alga, Haematococcus pluvialis, to protect cells from abiotic stress. However, information with regard to the acute (in minutes or hours) response of the cells to stress is scarce. In this study, two cDNAs encoding manganese superoxide dismutase (MnSOD) were cloned and their full length nucleotide sequences were obtained. Quantitative real-time RT-PCR analysis revealed a general increase in MnSOD transcriptions in response to various abiotic stressors, e.g., high light, excessive amounts of iron or sodium acetate, either singly or in combination. Meanwhile, four isoforms of MnSOD were detected by a nondenaturing polyacrylamide gel electrophoresis approach. In addition, an iron-containing superoxide dismutase (FeSOD) was detected in the cells using the same technique. Differential expressions of SOD isoenzymes under different abiotic stressors were also investigated and discussed, at both mRNA and protein levels.




Similar content being viewed by others
References
Alscher RG, Erturk N, Lenwood SH (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Armbrust E, Berges J, Bowler C, Green B, Martinez D, Putnam H, Zhou S, Allen E, Apt E, Bechner M, Brzezinski M, Chaal B, Chiovitti A, Davis A, Demarest M, Detter J, Glavina T, Goodstein D, Hadi M, Hellsten U, Hildebrand M, Jenkins B, Jurka J, Kapitonov V, Kröger N, Lau W, Lane T, Larimer F, Lippmeier J, Lucas S, Medina M, Montsant A, Obornik M, Parker M, Palenik B, Pazour G, Richardson P, Rynearson T, Saito M, Schwartz D, Thamatrakoln K, Valentin K, Vardi A, Wilkerson F, Rokhsa D (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer C, Mullineaux P (eds) Photooxidative Stresses in Plants: Causes and Amelioration. CRC Press, Boca Raton, pp 77–104
Asada K, Yoshikawa K, Takahashi M, Maeda Y, Enmanji K (1975) Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem 250:2801–7
Asada K, Kanematsu S, Uchida K (1977) Superoxide dismutases in photosynthetic organisms: absence of the cuprozinc enzyme in eukaryotic algae. Arch Biochem Biophys 179:243–256
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–94
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108:111–117
Boussiba S, Wang B, Yuan JP, Zarka A, Chen F (1999) Changes in pigments profile in the green alga Haematococcus pluvialis exposed to environmental stresses. Biotechnol Lett 21:601–604
Bowler C, Camp W, Vanmontagu M, Inze D (1994) Superoxide-dismutase in plants. CRC Crit Rev Plant Sci 13:199–218
Buchanan BB, Luan S (2005) Redox regulation in the chloroplast thylakoid lumen: a new frontier in photosynthesis research. J Exp Bot 56:1439–1447
Church S (1990) Manganese superoxide dismutase: nucleotide and deduced amino acid sequence of a cDNA encoding a new human transcript. Biochim Biophys Acta 1087:250–252
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Erturk HN (1999) Responses of superoxide dismutases to oxidative stress in Arabidopsis thaliana. In: Biology. Virginia Polytechnic Institute and State University, Blacksburg, VA, p 179
Fridovich I (1975) Superoxide dismutases. Annu Rev Biochem 44:147–159
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247:1–11
Gabriel O (1971) Locating enzymes on gels. In: Colowick SP, Kaplan NO (eds) Methods in Enzymology, vol 22. Academic, New York, p 578
Geigenberger P, Kolbe A, Tiessn A (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56:1469–1479
Grun S, Lindermayr C, Sell S (2006) Nitric oxide and gene regulation in plants. J Exp Bot 57:507–516
Hagen C, Braune W, Vogel K, Hader DP (1993) Functional aspects of secondary carotenoids in Haematococcus lacustris (Girod) Rostafinski (Volvocales) V. Influences on photomovement. Plant Cell Environ 16:991–995
Ho Y, Howard A, Crapo J (1991) Molecular structure of a functional rat gene for manganese-containing superoxide dismutase. Am J Respir Cell Mol Biol 4:278–286
Kaminaka H, Morita S, Tokumoto M, Yokoyama H, Masumura T, Tanaka K (1999) Molecular cloning and characterization of a cDNA for an iron-superoxide dismutase in rice (Oryza sativa L.). Biosci Biotech Biochem 63:302–308
Kanematsu S, Asada K (1990) Characteristic amino acid sequences of chloroplast and cytosol isozymes of CuZn-superoxide dismutase in spinach, rice and horsetail. Plant Cell Physiol 31:99–112
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9:627–640
Kitayama K (1994) Molecular analysis of photooxidative stress responses in Chlamydomonas reinhardtii. Thesis, Indiana University, Bloomington
Kliebenstein DJ, Monde RA, Last RL (1998) Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol 118:637–650
Kobayashi M, Kakizono T, Nagai S (1991) Astaxanthin production by a green-alga, Haematococcus pluvialis accompanied with morphological-changes in acetate media. J Ferment Bioeng 71:335–339
Kobayashi M, Kakizono T, Nishio N, Nagai S, Kurimura Y, Tsuji Y (1997) Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol 48:351–356
Kwon SJ, Choi EY, Choi YJ (2006) Proteomics studies of post-translational modifications in plants. J Exp Bot 57:1547–1551
Li YT, Sommerfeld M, Chen F, Hu Q (2008) Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol 165:1783–1797
Lu F, Vonshak A, Zarka A, Boussiba S (1998) Does astaxanthin protect Haematococcus against light damage? Z Naturforsch 53c:93–100
Matsuzaki M, Misumi O, Shin-I T, Maruyama S, Takahara M, Miyagishima SY, Mori T, Nishida K, Yagisawa F, Nishida K, Yoshida Y, Nishimura Y, Nakao S, Kobayashi T, Momoyama Y, Higashiyama T, Minoda A, Sano M, Nomoto H, Oishi K, Hayashi H, Ohta F, Nishizaka S, Haga S, Miura S, Morishita T, Kabeya Y, Terasawa K, Suzuki Y, Ishii Y, Asakawa S, Takano H, Ohta N, Kuroiwa H, Tanaka K, Shimizu N, Sugano S, Sato N, Nozaki H, Ogasawara N, Kohara Y, Kuroiwa T (2004) Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428:653–657
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Mur LAJ, Tim L, Carver W (2006) No way to live: The various roles of nitric oxide in plant-pathogen interactions. J Exp Bot 57:489–505
Parker M, Blake C, Barra D, Bossa F, Schinina M, Bannister W, Bannister J (1987) Structural identity between the iron- and manganese-containing superoxide dismutases. Protein Eng 1:393–397
Perl-Treves R, Perl A (2002) Oxidative stress: an introduction. In: Van Montagu M, Inzé D (eds) Oxidative stress in plants. Taylor & Francis, London, pp 1–32
Sakamoto A, Nosaka Y, Tanaka K (1993) Cloning and sequencing analysis of a complementary DNA for manganese-superoxide dismutase from rice (Oryza sativa L.). Plant Physiol 103:1477–1478
Sakurai H, Kusumoto N, Kitayama K, Robert TK (1993) Isozymes of superoxide dismutase in Chlamydomonas and purification of one of the major isozymes containing Fe. Plant Cell Physiol 34:1133–1137
Shaish A, Avron M, Pick U, Ben-Amotz A (1993) Are active oxygen species involved in induction of β-carotene in Dunaliella bardawil? Planta 190:363–8
Steinbrenner J, Linden H (2001) Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol 125:810–817
Steinbrenner J, Linden H (2003) Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol 52:343–56
Tsang EW, Bowler C, Hérouart D, Van Camp W, Villarroel R, Genetello C, Van Montagu M, Inzé D (1991) Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell 3:783–92
Ukeda H, Sarker AK, Kawana D, Sawamura M (1999) Flow-injection assay of superoxide dismutase based on the reduction of highly water-soluble tetrazolium. Anal Sci 15:353–357
Wang JX, Shi ZX, Xu XD (2004a) Residual plastids of bleached mutants of Euglena gracilis and their effects on the expression of nucleus-encoded genes. Prog Nat Sci 14:213–217
Wang JX, Sommerfeld M, Hu Q (2009) Occurrence and environmental stress responses of two plastid terminal oxidases in Haematococcus pluvialis (Chlorophyceae). Planta 230:191–203
Wang JX, Zhang XZ, Chen YS, Sommerfeld M, Hu Q (2008) Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii. Chemosphere 73:1121–1128
Wang S, Chen F, Sommerfeld M, Hu Q (2004b) Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcus pluvialis (Chlorophyceae). Planta 220:17–29
Wuerges J, Lee J, Yim Y, Yim H, Kang S, Carugo K (2004) Crystal structure of nickel-containing superoxide dismutase reveals another type of active site. Proc Natl Acad Sci USA 101:8569–8574
Youn HD, Kim E, Roe J, Hah Y, Kang S (1996) A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochem J 318:889–896
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38:325–331
Zhu D, Scandalios J (1993) Maize mitochondrial manganese superoxide dismutases are encoded by a differentially expressed multigene family. Proc Natl Acad Sci USA 90:9310–9314
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Sommerfeld, M. & Hu, Q. Cloning and expression of isoenzymes of superoxide dismutase in Haematococcus pluvialis (Chlorophyceae) under oxidative stress. J Appl Phycol 23, 995–1003 (2011). https://doi.org/10.1007/s10811-010-9631-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9631-6