Abstract
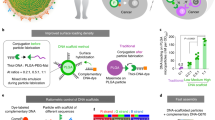
Adoptive cell therapy (ACT) is an immunotherapy strategy for cancer that has seen widespread clinical success. During ACT, patient-derived lymphocytes are stimulated with the antigen of interest ex vivo, proliferated, then returned to the patient to initiate an antigen-specific antitumor response. While effective, this process is resource-intensive and logistically impossible for many patients. Particulate artificial antigen presenting cells (aAPCs) offer a potential “off-the-shelf” alternative to ex vivo ACT. While particulate aAPCs perform well in vitro, they have had limited success in vivo due to poor bioavailability after injection. Barriers to bioavailability include rapid clearance, unfavorable biodistribution, and inadequate interactions with CD8+ T cells at sites of interest. Biomaterial properties such as elasticity have been shown to vastly impact the bioavailability and particle-cell interactions, but this has yet to be investigated in the context of aAPCs for in vivo T-cell stimulation. Previous literature likewise indicates that biomaterial properties, especially elasticity, can modulate T-cell activation in vitro. With the goal of creating a more biomimetic, next-generation particulate aAPC, we developed a poly(ethylene) glycol hydrogel particle platform with tunable elasticity to investigate the impact of elasticity on antigen-specific T cell activation for in vivo adoptive transfer. Using this knowledge, we were able to gain more precise control over in vivo T cell activation and investigate possible mechanisms including the effects of aAPC elasticity on T cell binding, macrophage uptake, and the protein corona.

Similar content being viewed by others
References
Raskov, H.; Orhan, A.; Christensen, J. P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367.
Bailey, S. R.; Berger, T. R.; Graham, C.; Larson, R. C.; Maus, M. V. Four challenges to CAR T cells breaking the glass ceiling. Eur. J. Immunol. 2023, 53, 2250039.
Wang, C.; Sun, W. J.; Ye, Y. Q.; Bomba, H. N.; Gu, Z. Bioengineering of artificial antigen presenting cells and lymphoid organs. Theranostics 2017, 7, 3504–3516.
Est-Witte, S. E.; Livingston, N. K.; Omotoso, M. O.; Green, J. J.; Schneck, J. P. Nanoparticles for generating antigen-specific T cells for immunotherapy. Semin. Immunol. 2021, 56, 101541.
Bandola-Simon, J.; Roche, P. A. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol. Immunol. 2019, 113, 31–37.
Zhang, Z.; Liu, S. S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T cell dysfunction and exhaustion in cancer. Front. Cell Dev. Biol. 2020, 8, 17.
Ben-Akiva, E.; Witte, S. E.; Meyer, R. A.; Rhodes, K. R.; Green, J. J. Polymeric micro-and nanoparticles for immune modulation. Biomater. Sci. 2019, 7, 14–30.
Meyer, R. A.; Sunshine, J. C.; Perica, K.; Kosmides, A. K.; Aje, K.; Schneck, J. P.; Green, J. J. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 2016, 11, 1519–1525.
Meyer, R.; Sunshine, J.; Green, J. J. Biomimetic particles as therapeutics. Trends. Biotechnol. 2015, 33, 514–524
Ben-Akiva, E.; Meyer, R. A.; Wilson, D. R.; Green, J. J. Surface engineering for lymphocyte programming. Adv. Drug Deliv. Rev. 2017, 114, 102–115.
Rhodes, K. R.; Isser, A.; Hickey, J. W.; Ben-Akiva, E.; Meyer, R. A.; Kosmides, A. K.; Livingston, N. K.; Tzeng, S. Y.; Schneck, J. P.; Green, J. J. Biodegradable cationic polymer blends for fabrication of enhanced artificial antigen presenting cells to treat melanoma. ACS Appl. Mater. Interfaces 2021, 13, 7913–7923.
Harrison, D. L.; Fang, Y.; Huang, J. T-cell mechanobiology: Force sensation, potentiation, and translation. Front. Phys. 2019, 7, 45.
Du, H. X.; Bartleson, J. M.; Butenko, S.; Alonso, V.; Liu, W. F.; Winer, D. A.; Butte, M. J. Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. 2023, 23, 174–188.
Meng, K. P.; Majedi, F. S.; Thauland, T. J.; Butte, M. J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 2020, 217, e20200053.
Platzman, I.; Kannenberg, G.; Janiesch, J. W.; Matić, J.; Spatz, J. PEG-based antigen-presenting cell surrogates for immunological applications. In Soft Matter Nanotechnology: From Structure to Function. Chen, X.; Fuchs, H., Eds.; Wiley-VCH: Weinheim, 2015; pp 187–215.
Hickey, J. W.; Dong, Y.; Chung, J. W.; Salathe, S. F.; Pruitt, H. C.; Li, X. W.; Chang, C.; Fraser, A. K.; Bessell, C. A.; Ewald, A. J. et al. Engineering an artificial T-cell stimulating matrix for immunotherapy. Adv. Mater. 2019, 31, 1807359.
Saitakis, M.; Dogniaux, S.; Goudot, C.; Bufi, N.; Asnacios, S.; Maurin, M.; Randriamampita, C.; Asnacios, A.; Hivroz, C. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. eLife 2017, 6, e23190.
Guo, P.; Liu, D. X.; Subramanyam, K.; Wang, B. R.; Yang, J.; Huang, J.; Auguste, D. T.; Moses, M. A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 130.
Anselmo, A. C.; Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. 2017, 108, 51–67.
Anselmo, A. C.; Zhang, M. W.; Kumar, S.; Vogus, D. R.; Menegatti, S.; Helgeson, M. E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015, 9, 3169–3177.
Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D. Y.; Gao, H. J.; Zhao, C. X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano 2019, 13, 7410–7424.
Cui, J. W.; De Rose, R.; Alt, K.; Alcantara, S.; Paterson, B. M.; Liang, K.; Hu, M.; Richardson, J. J.; Yan, Y.; Jeffery, C. M. et al. Engineering poly(ethylene glycol) particles for improved biodistribution. ACS Nano 2015, 9, 1571–1580.
Van Thienen, T. G.; Demeester, J.; De Smedt, S. C. Screening poly(ethyleneglycol) micro- and nanogels for drug delivery purposes. Int. J. Pharm. 2008, 351, 174–185.
Sunshine, J. C.; Perica, K.; Schneck, J. P.; Green, J. J. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials 2014, 35, 269–277.
Akhtar, R.; Sherratt, M. J.; Cruickshank, J. K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105.
Doshi, N.; Zahr, A. S.; Bhaskar, S.; Lahann, J.; Mitragotri, S. Red blood cell-mimicking synthetic biomaterial particles. Proc. Natl. Acad. Sci. USA 2009, 106, 21495–21499.
Overwijk, W. W.; Tsung, A.; Irvine, K. R.; Parkhurst, M. R.; Goletz, T. J.; Tsung, K.; Carroll, M. W.; Liu, C. L.; Moss, B.; Rosenberg, S. A. et al. gp100/pmel 17 is a murine tumor rejection antigen: Induction of “Self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998, 188, 277–286.
Waldman, A. D.; Fritz, J. M.; Lenardo, M. J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668.
Wauters, A. C.; Scheerstra, J. F.; Vermeijlen, I. G.; Hammink, R.; Schluck, M.; Woythe, L.; Wu, H. L.; Albertazzi, L.; Figdor, C. G.; Tel, J. et al. Artificial antigen-presenting cell topology dictates T Cell activation. ACS Nano 2022, 16, 15072–15085.
Majedi, F. S.; Hasani-Sadrabadi, M. M.; Thauland, T. J.; Li, S.; Bouchard, L. S.; Butte, M. J. Augmentation of T-cell activation by oscillatory forces and engineered antigen-presenting cells. Nano Lett. 2019, 19, 6945–6954.
Ye, L. L.; Wei, X. S.; Zhang, M.; Niu, Y. R.; Zhou, Q. The significance of tumor necrosis factor receptor type II in CD8+ regulatory T cells and CD8+ effector T cells. Front. Immunol. 2018, 9, 583.
Majedi, F. S.; Hasani-Sadrabadi, M. M.; Thauland, T. J.; Li, S.; Bouchard, L. S.; Butte, M. J. T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 2020, 252, 120058.
Mishra, R. K.; Ahmad, A.; Vyawahare, A.; Alam, P.; Khan, T. H.; Khan, R. Biological effects of formation of protein corona onto nanoparticles. Int. J. Biol. Macromol. 2021, 175, 1–18.
Li, H. M.; Wang, Y.; Tang, Q.; Yin, D.; Tang, C.; He, E.; Zou, L.; Peng, Q. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021, 129, 57–72.
Tomak, A.; Cesmeli, S.; Hanoglu, B. D.; Winkler, D.; Karakus, C. O. Nanoparticle-protein corona complex: Understanding multiple interactions between environmental factors, corona formation, and biological activity. Nanotoxicology 2021, 15, 1331–1357.
Panico, S.; Capolla, S.; Bozzer, S.; Toffoli, G.; Dal Bo, M.; Macor, P. Biological features of nanoparticles: Protein corona formation and interaction with the immune system. Pharmaceutics 2022, 14, 2605.
Tengjisi; Hui, Y.; Fan, Y. Y.; Zou, D.; Talbo, G. H.; Yang, G. Z.; Zhao, C. X. Influence of nanoparticle mechanical property on protein corona formation. J. Colloid Interface Sci. 2022, 606, 1737–1744.
Partikel, K.; Korte, R.; Stein, N. C.; Mulac, D.; Herrmann, F. C.; Humpf, H. U.; Langer, K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80.
Yu, F. C.; Teo, G. C.; Kong, A. T.; Haynes, S. E.; Avtonomov, D. M.; Geiszler, D. J.; Nesvizhskii, A. I. Identification of modified peptides using localization-aware open search. Nat. Commun. 2020, 11, 4065.
Polasky, D. A.; Yu, F. C.; Teo, G. C.; Nesvizhskii, A. I. Fast and comprehensive N- and O-glycoproteomics analysis with MSFragger-Glyco. Nat. Methods 2020, 17, 1125–1132.
Teo, G. C.; Polasky, D. A.; Yu, F. C.; Nesvizhskii, A. I. Fast deisotoping algorithm and its implementation in the MSFragger search engine. J. Proteome Res. 2021, 20, 498–505.
Yu, F. C.; Haynes, S. E.; Teo, G. C.; Avtonomov, D. M.; Polasky, D. A.; Nesvizhskii, A. I. Fast quantitative analysis of timsTOF PASEF data with MSFragger and IonQuant. Mol. Cell Proteomics 2020, 19, 1575–1585.
Chang, H. Y.; Kong, A. T.; Da Veiga Leprevost, F.; Avtonomov, D. M.; Haynes, S. E.; Nesvizhskii, A. I. Crystal-C: A computational tool for refinement of open search results. J. Proteome Res. 2020, 19, 2511–2515.
Geiszler, D. J.; Kong, A. T.; Avtonomov, D. M.; Yu, F. C.; Da Veiga Leprevost, V.; Nesvizhskii, A. I. PTM-Shepherd: Analysis and summarization of post-translational and chemical modifications from open search results. Mol. Cell Proteomics 2021, 20, 100018.
Tsou, C. C.; Avtonomov, D.; Larsen, B.; Tucholska, M.; Choi, H.; Gingras, A. C.; Nesvizhskii, A. I. DIA-Umpire: Comprehensive computational framework for data-independent acquisition proteomics. Nat. Methods 2015, 12, 258–264.
Gao, B. B.; Zhu, J.; Negi, S.; Zhang, X. M.; Gyoneva, S.; Casey, F.; Wei, R.; Zhang, B. H. Quickomics: Exploring omics data in an intuitive, interactive and informative manner. Bioinformatics 2021, 37, 3670–3672.
Parhami-Seren, B.; Viswanathan, M.; Strong, R. K.; Margolies, M. N. Structural analysis of mutants of high-affinity and low-affinity p-azophenylarsonate-specific antibodies generated by alanine scanning of heavy chain complementarity-determining region 2. J. Immunol. 2001, 167, 5129–5135.
Siegelman, M.; Capra, J. D. Complete amino acid sequence of light chain variable regions derived from five monoclonal anti-p-azophenylarsonate antibodies differing with respect to a crossreactive idiotype. Proc. Natl. Acad. Sci. USA 1981, 78, 7679–7683.
Chinen, A. B.; Guan, C. M.; Ko, C. H.; Mirkin, C. A. The impact of protein corona formation on the macrophage cellular uptake and biodistribution of spherical nucleic acids. Small 2017, 13, 1603847.
Santiago-Sánchez, G. S.; Pita-Grisanti, V.; Quiñones-Díaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejía, P. E. Biological functions and therapeutic potential of Lipocalin 2 in cancer. Int. J. Mol. Sci. 2020, 21, 4365.
Warszawska, J. M.; Gawish, R.; Sharif, O.; Sigel, S.; Doninger, B.; Lakovits, K.; Mesteri, I.; Nairz, M.; Boon, L.; Spiel, A. et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J. Clin. Invest. 2013, 123, 3363–3372.
Zumwalde, N. A.; Domae, E.; Mescher, M. F.; Shimizu, Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J. Immunol. 2013, 191, 3681–3693.
Cox, M. A.; Barnum, S. R.; Bullard, D. C.; Zajac, A. J. ICAM-1-dependent tuning of memory CD8 T-cell responses following acute infection. Proc. Natl. Acad. Sci. USA 2013, 110, 1416–1421.
Zhong, H. H.; Lin, H. T.; Pang, Q. N.; Zhuang, J. L.; Liu, X. L.; Li, X. L.; Liu, J. H.; Tang, J. Macrophage ICAM-1 functions as a regulator of phagocytosis in LPS induced endotoxemia. Inflamm. Res. 2021, 70, 193–203.
Crompton, J. G.; Narayanan, M.; Cuddapah, S.; Roychoudhuri, R.; Ji, Y.; Yang, W.; Patel, S. J.; Sukumar, M.; Palmer, D. C.; Peng, W. et al. Lineage relationship of CD8+ T cell subsets is revealed by progressive changes in the epigenetic landscape. Cell. Mol. Immunol. 2015, 13, 502–513.
Cho, J.; Seo, J.; Lim, C. H.; Yang, L.; Shiratsuchi, T.; Lee, M. H.; Chowdhury, R. R.; Kasahara, H.; Kim, J. S.; Oh, S. P. Mitochondrial ATP transporter Ant2 depletion impairs erythropoiesis and B lymphopoiesis. Cell Death Differ. 2015, 22, 1437–1450.
Saragovi, A.; Abramovich, I.; Omar, I.; Arbib, E.; Toker, O.; Gottlieb, E.; Berger, M. Systemic hypoxia inhibits T cell response by limiting mitobiogenesis via matrix substrate-level phosphorylation arrest. eLife 2020, 9, e56612.
Moon, J. S.; Da Cunha, F. F.; Huh, J. Y.; Andreyev, A. Y.; Lee, J.; Mahata, S. K.; Reis, F. C. G.; Nasamran, C. A.; Lee, Y. S. ANT2 drives proinflammatory macrophage activation in obesity. JCI Insight 2021, 22, e147033
Beck, I. M.; Rückert, R.; Brandt, K.; Mueller, M. S.; Sadowski, T.; Brauer, R.; Schirmacher, P.; Mentlein, R.; Sedlacek, R. MMP19 is essential for T cell development and T cell-mediated cutaneous immune responses. PLoS One 2008, 3, e2343
Matysiak-Kucharek, M.; Czajka, M.; Sawicki, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Effect of nanoparticles on the expression and activity of matrix metalloproteinases. Nanotechnol. Rev. 2018, 7, 541–553.
Huang, W. C.; Sala-Newby, G. B.; Susana, A.; Johnson, J. L.; Newby, A. C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One 2012, 7, e42507.
Park, E. J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitro 2010, 24, 872–878.
Zuidema, J. M.; Rivet, C. J.; Gilbert, R. J.; Morrison, F. A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073.
Abramoff, M. D.; Magalhaes, P. J.; Ram, S. J. Image processing with ImageJ. Biophoton. Int. 2004, 11, 36–42.
Acknowledgements
The authors would like to thank the NIH for support of this research (P41EB028239). This material is based upon work subsidized by the National Science Foundation Graduate Research Fellowship (Nos. DGE-1746891 (SEW) and DGE-1746891 (SRS)). S. E. W. thanks the ARCS Metro Washington Chapter and the Siebel Scholars Foundation. Figure diagrams were created with biorender.com. The authors would also like to thank Vance Soares for his assistance with training in microscopy.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2024_6589_MOESM1_ESM.pdf
Particle elasticity influences polymeric artificial antigen presenting cell effectiveness in vivo via CD8+ T cell activation, macrophage uptake, and the protein corona
Rights and permissions
About this article
Cite this article
Est-Witte, S.E., Shannon, S.R., Gong, D.H. et al. Particle elasticity influences polymeric artificial antigen presenting cell effectiveness in vivo via CD8+ T cell activation, macrophage uptake, and the protein corona. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6589-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6589-2