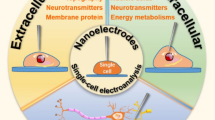
Abstract
Cells are the basic unit of life. Electrochemical analysis of single cells/organelles is essential for uncovering the molecular mechanisms of physiological and pathological processes that are difficult to elucidate on a larger scale. This paper provides an overview of the commonly used fabrication methods for micro/nanoelectrodes applied in the investigations of single cells/organelles as well as the corresponding electrochemical measurements over the last four years including extracellular measurement, combination of extra and intracellular measurement, intracellular reactive oxygen species and reactive nitrogen species (ROS/RNS) measurement, and isolated organelles measurement.

Similar content being viewed by others
References
Amatore, C.; Arbault, S.; Guille, M.; Lemaître, F. Electrochemical monitoring of single cell secretion: Vesicular exocytosis and oxidative stress. Chem. Rev. 2008, 108, 2585–2621.
Chen, G.; Ewing, A. G. Chemical analysis of single cells and exocytosis. Crit Rev Neurobiol. 1997, 11, 59–90.
Zhang, X. W.; Hatamie, A.; Ewing, A. G. Nanoelecroochemical analysis inside a single living cell. Curr. Opin. Electrochem. 2020, 22, 94–101.
Wightman, R. M. Probing cellular chemistry in biological systems with microelectrodes. Science 2006, 311, 1570–1574.
Schulte, A.; Schuhmann, W. Siggle-cell microelectrochemistry. Angew. Chem., Int. Ed. 2007, 46, 8760–8777.
Wightman, R. M.; Jankowski, J. A.; Kennedy, R. T.; Kawagoe, K. T.; Schroeder, T. J.; Leszczyszyn, D. J.; Near, J. A.; Diliberto, E. J. Jr.; Viveros, O. H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. USA 1991, 88, 10754–10758.
Xu, C.; Jiang, Y.; Yu, P.; Mao, L. Q. Brain electrochemistry. J. Electrochem. 2022, 28, 2108551.
Liu, Y. D.; Li, J. R.; Zhang, L. M.; Tian, Y. An aptamer-based microelectrode with tunable linear range for monitoring of K+ in the living mouse brain. J. Electrochem. 2023, 29, 2218004.
Li, Y. T.; Zhang, S. H.; Wang, L.; Xiao, R. R.; Liu, W.; Zhang, X. W.; Zhou, Z.; Amatore, C.; Huang, W. H. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angew. Chem., Int. Ed. 2014, 55, 12456–12460.
Zhang, X. W.; Qiu, Q. F.; Jiang, H.; Zhang, F. L.; Liu, Y. L.; Amatore, C.; Huang, W. H. Real-time intracellular measurements of ROS and RNS in living cells with single core-shell nanowire electrodes. Angew. Chem., Int. Ed. 2017, 56, 12997–13000.
Clausmeyer, J.; Wilde, P.; Löffler, T.; Ventosa, E.; Tschulik, K.; Schuhmann, W. Detection of individual nanoparticle impacts using etched carbon nanoelectrodes. Electrochem. Commun. 2016, 73, 67–70.
Clausmeyer, J.; Masa, J.; Ventosa, E.; öhl, D.; Schuhmann, W. Nanoelectrodes reveal the electrochemistry of single nickelhydroxide nanoparticles. Chem. Commun. 2016, 52, 2408–2411.
Hu, K. K.; Wang, Y. X.; Cai, H. J.; Mirkin, M. V.; Gao, Y.; Friedman, G.; Gogotsi, Y. Open carbon nanopipettes as resistive-pulse sensors, rectification sensors, and electrochemical nanoprobes. Anal. Chem. 2014, 86, 8897–8901.
Tanwar, A.; Gandhi, H. A.; Kushwaha, D.; Bhattacharya, J. A review on microelectrode array fabrication techniques and their applications. Mater. Today Chem. 2022, 26, 101153.
Ranjbari, E.; Taleat, Z.; Mapar, M.; Aref, M.; Dunevall, J.; Ewing, A. Direct measurement of total vesicular catecholamine content with electrochemical microwell arrays. Anal. Chem. 2020, 92, 11325–11331.
Kawagoe, K. T.; Jankowski, J. A.; Wightman, R. M. Etched carbone fiber electrodes as amperometric detectors of catecholamine secretion from isolated biological cells. Anal. Chem. 1991, 65, 1589–1594.
Li, X. C.; Majdi, S.; Dunevall, J.; Fathali, H.; Ewing, A. G. Quantitative measurement of transmitters in individual vesicles in the cytoplasm of single cells with nanotip electrodes. Angew. Chem., Int. Ed. 2015, 54, 11978–11982.
Strein, T. G.; Ewing, A. G. Characterization of submicron-sized carbon electrodes insulated with a phenol-allylphenol copolymer. Anal. Chem. 1992, 64, 1368–1373.
Huang, W. H.; Pang, D. W.; Tong, H.; Wang, Z. L.; Cheng, J. K. A method for the fabrication of low-noise carbon fiber nanoelectrodes. Anal. Chem. 2001, 75, 1048–1052.
Strand, A. M.; Venton, B. J. Flame etching enhances the sensitivity of carbon-fiber microelectrodes. Anal. Chem. 2008, 80, 3708–3715.
Liao, Q. L.; Jiang, H.; Zhang, X. W.; Qiu, Q. F.; Tang, Y.; Yang, X. K.; Liu, Y. L.; Huang, W. H. A single nanowire sensor for intracellular glucose detection. Nanoscale 2019, 11, 10702–10708.
Jiang, H.; Zhang, X. W.; Liao, Q. L.; Wu, W. T.; Liu, Y. L.; Huang, W. H. Electrochemical monitoring of paclitaxel-induced ROS release from mitochondria inside single cells. Small 2019, 15, 1901787.
Yang, X. K.; Zhang, F. L.; Wu, W. T.; Tang, Y.; Yan, J.; Liu, Y. L.; Amatore, C.; Huang, W. H. Quantitative Nano-amperometric measurement of intravesicular glutamate content and its sub-quantal release by living neurons. Angew. Chem., Int. Ed. 2021, 60, 15803–15808.
Kim, Y. T.; Scarnulis, D. M.; Ewing, A. G. Carbon-ring electrodes with 1-µm tip diameter. Anal. Chem. 1986, 58, 1782–1786.
McKelvey, K.; Nadappuram, B. P.; Actis, P.; Takahashi, Y.; Korchev, Y. E.; Matsue, T.; Robinson, C.; Unwin, P. R. Fabrication, characterization, and functionalization of dual carbon electrodes as probes for scanning electrochemical microscopy (SECM). Anal. Chem. 2013, 85, 7519–7526.
McNally, M.; Wong, D. K. Y. An in vivo probe based on mechanically strong but structurally small carbon electrodes with an appreciable surface area. Anal. Chem. 2001, 73, 4793–4800.
Nadappuram, B. P.; McKelvey, K.; Al Botros, R.; Colburn, A. W.; Unwin, P. R. Fabrication and characterization of dual function nanoscale pH-scanning ion conductance microscopy (SICM) probes for high resolution pH mapping. Anal. Chem. 2013, 85, 8070–8074.
Takahashi, Y.; Shevchuk, A. I.; Novak, P.; Zhang, Y. J.; Ebejer, N.; Macpherson, J. V.; Unwin, P. R.; Pollard, A. J.; Roy, D.; Clifford, C. A. et al. Multifunctional nanoprobes for nanoscale chemical imaging and localized chemical delivery at surfaces and interfaces. Angew. Chem., Int. Ed. 2011, 56, 9638–9642.
Wong, D. K. Y.; Xu, L. Y. F. Voltammetric studies of carbon disk electrodes with submicrometer-sized structural diameters. Anal. Chem. 1995, 67, 4086–4090.
Hu, K. K.; Gao, Y.; Wang, Y. X.; Yu, Y.; Zhao, X.; Rotenberg, S. A.; Gökmeşe, E.; Mirkin, M. V.; Friedman, G.; Gogotsi, Y. Platinized carbon nanoelectrodes as potentiometric and amperometric SECM probes. J. Solid State Electrochem. 2013, 17, 2971–2977.
Singhal, R.; Bhattacharyya, S.; Orynbayeva, Z.; Vitol, E.; Friedman, G.; Gogotsi, Y. Small diameter carbon nanopipettes. Nanotechnology 2010, 21, 015304.
Vitol, E. A.; Schrlau, M. G.; Bhattacharyya, S.; Ducheyne, P.; Bau, H. H.; Friedman, G.; Gogotsi, Y. Effects of deposition conditions on the structure and chemical properties of carbon nanopipettes. Chem. Vap. Deposition 2009, 15, 204–208.
Yu, Y.; Noël, J. M.; Mirkin, M. V.; Gao, Y.; Mashtalir, O.; Friedman, G.; Gogotsi, Y. Carbon pipette-based electrochemical nanosampler. Anal. Chem. 2014, 86, 3365–3372.
Pan, R. R.; Xu, M. C.; Burgess, J. D.; Jiang, D. C.; Chen, H. Y. Direct electrochemical observation of glucosidase activity in isolated single lysosomes from a living cell. Proc. Natl. Acad. Sci. USA 2018, 115, 4087–4092.
Pan, R. R.; Xu, M. C.; Jiang, D. C.; Burgess, J. D.; Chen, H. Y. Nanokit for single-cell electrochemical analyses. Proc. Natl. Acad. Sci. USA 2016, 113, 11436–11440.
Jiao, Y. T.; Jiang, H.; Wu, W. T.; Qi, Y. T.; Wen, M. Y.; Yang, X. K.; Kang, Y. R.; Zhang, X. W.; Amatore, C.; Huang, W. H. Dual-channel nanoelectrochemical sensor for monitoring intracellular ROS and NADH kinetic variations of their concentrations. Biosens. Bioelectron. 2023, 222, 114928.
Qi, Y. T.; Jiang, H.; Wu, W. T.; Zhang, F. L.; Tian, S. Y.; Fan, W. T.; Liu, Y. L.; Amatore, C.; Huang, W. H. Homeostasis inside single activated phagolysosomes: Quantitative and selective measurements of submillisecond dynamics of reactive oxygen and nitrogen species production with a nanoelectrochemical sensor. J. Am. Chem. Soc. 2022, 144, 9723–9733.
Huang, X. J.; O’Mahony, A. M.; Compton, R. G. Microelectrode arrays for electrochemistry: Approaches to fabrication. Small 2009, 5, 776–788.
Zaouk, R.; Park, B. Y.; Madou, M. J. Introduction to microfabrication techniques. In Microfluidic Techniques: Reviews and Protocols. Minteer, S. D., Ed.; Humana Press: Totowa, 2006; pp 5–15.
Abbott, J.; Ye, T. Y.; Qin, L.; Jorgolli, M.; Gertner, R. S.; Ham, D.; Park, H. CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging. Nat. Nanotechnol. 2017, 12, 460–466.
Zheng, T. Y.; Zhang, Z. Z.; Zhu, R.; Sun, D. A microelectrode array chip for osteogenic differentiation of mesenchymal stem cells under electrical stimulation. Lab Chip 2020, 20, 373–383.
Zheng, T. Y.; Zhang, Z. Z.; Zhu, R. Flexible trapping and manipulation of single cells on a chip by modulating phases and amplitudes of electrical signals applied onto microelectrodes. Anal. Chem. 2019, 91, 4479–4487.
Phan, N. T. N.; Li, X. C.; Ewing, A. G. Measuring synaptic vesicles using cellular electrochemistry and nanoscale molecular imaging. Nat. Rev. Chem. 2017, 1, 0048.
Wang, M. Y.; Liu, Y. Y.; Du, J. C.; Zhou, J. L.; Cao, L. J.; Li, X. C. Cisplatin inhibits neurotransmitter release during exocytosis from single chromaffin cells monitored with single cell amperometry. Electroanalysis 2022, 34, 981–986.
He, X. L.; Ewing, A. G. Concentration of stimulant regulates initial exocytotic molecular plasticity at single cells. Chem. Sci. 2022, 13, 1815–1822.
He, X. L.; Ewing, A. G. Counteranions in the stimulation solution alter the dynamics of exocytosis consistent with the hofmeister series. J. Am. Chem. Soc. 2020, 142, 12591–12595.
He, X.; Ewing, A. G. Hofmeister series: From aqueous solution of biomolecules to single cells and nanovesicles. ChemBioChem 2023, 24, e202200694.
Gu, C. Y.; Zhang, X. W.; Ewing, A. G. Comparison of disk and Nanotip electrodes for measurement of single-cell amperometry during exocytotic release. Anal. Chem. 2020, 92, 10268–10273.
McCarty, G. S.; Dunaway, L. E.; Denison, J. D.; Sombers, L. A. Neurotransmitter readily escapes detection at the opposing microelectrode surface in typical amperometric measurements of exocytosis at single cells. Anal. Chem. 2022, 94, 9548–9556.
Jia, R.; Rotenberg, S. A.; Mirkin, M. V. Electrochemical resistive-pulse sensing of extracellular vesicles. Anal. Chem. 2022, 94, 12614–12620.
Hu, K. K.; Le Vo, K. L.; Wang, F.; Zhang, X.; Gu, C. Y.; Fang, N.; Phan, N. T. N.; Ewing, A. G. Single exosome amperometric measurements reveal encapsulation of chemical messengers for intercellular communication. J. Am. Chem. Soc. 2023, 145, 11499–11503.
Frebel, H.; Chemnitius, G. C.; Cammann, K.; Kakerow, R.; Rospert, M.; Mokwa, W. Multianalyte sensor for the simultaneous determination of glucose, L-lactate and uric acid based on a microelectrode array. Sens. Actuators B: Chem. 1997, 43, 87–93.
Chuang, M. C.; Lai, H. Y.; Annie Ho, J. A.; Chen, Y. Y. Multifunctional microelectrode array (mMEA) chip for neural-electrical and neural-chemical interfaces: Characterization of comb interdigitated electrode towards dopamine detection. Biosens. Bioelectron. 2013, 41, 602–607.
Yang, L. H.; Liu, X. B.; Yin, B.; Deng, X. X.; Lin, X. T.; Song, J.; Wu, S. High-throughput and real-time monitoring of single-cell extracellular pH based on polyaniline microarrays. Anal. Chem. 2021, 93, 13852–13860.
Wang, N. N.; Ao, H.; Xiao, W. C.; Chen, W. W.; Li, G. M.; Wu, J.; Ju, H. X. Confined electrochemiluminescence imaging microarray for high-throughput biosensing of single cell-released dopamine. Biosens. Bioelectron. 2022, 261, 113959.
Tian, Z. Y.; Qin, X.; Shao, F. Y.; Li, X. X.; Wang, Z.; Liu, S. Q.; Wu, Y. F. Electrofluorochromic imaging analysis of dopamine release from living PC12 cells with bipolar nanoelectrodes array. Chin. Chem. Lett. 2023, 34, 107656.
Guo, X. L.; Zhu, R. Controllable in-situ cell electroporation with cell positioning and impedance monitoring using micro electrode array. Sci. Rep. 2016, 6, 31392.
Neumann, E.; Tönsing, K.; Siemens, P. Perspectives for microelectrode arrays for biosensing and membrane electroporation. Bioelectrochemistry 2000, 51, 125–132.
Chang, L. Q.; Li, L.; Shi, J. F.; Sheng, Y.; Lu, W.; Gallego-Perez, D.; Lee, L. J. Micro-/nanoscale electroporation. Lab Chip 2016, 16, 4047–4062.
Zhang, Z. Z.; Zheng, T. Y.; Zhu, R. Single-cell individualized electroporation with real-time impedance monitoring using a microelectrode array chip. Microsyst. Nanoeng. 2020, 6, 81.
Breckenridge, L. J.; Wilson, R. J. A.; Connolly, P.; Curtis, A. S. G.; Dow, J. A. T.; Blackshaw, S. E.; Wilkinson, C. D. W. Advantages of using microfabricated extracellular electrodes for in vitro neuronal recording. J. Neurosci. Res. 1995, 42, 266–276.
Taleat, Z.; Larsson, A.; Ewing, A. G. Anticancer drug tamoxifen affects catecholamine transmitter release and storage from single cells. ACS Chem. Neurosci. 2019, 10, 2060–2069.
Zhou, J. L.; Zhang, J.; Cao, L. J.; Liu, Y. Y.; Liu, L. Y.; Liu, C. L.; Li, X. C. Ginsenoside Rg1 modulates vesicular dopamine storage and release during exocytosis revealed with single-vesicle electrochemistry. Chem. Commun. 2023, 59, 3087–3090.
Aref, M.; Ranjbari, E.; Romiani, A.; Ewing, A. G. Intacellular injection of phospholipids directly alters exocytosis and the fraction of chemical release in chromaffin cells as measured by Nanoe electrochemistry. Chem. Sci. 2020, 11, 11869–11876.
Gu, C. Y.; Ewing, A. G. Simultaneous detection of vesicular content and exocytotic release with two electrodes in and at a single cell. Chem. Sci. 2021, 12, 7393–7400.
Hu, K. K.; Le Vo, K. L.; Hatamie, A.; Ewing, A. G. Quantifying intracellular single vesicular catecholamine concentration with open carbon nanopipettes to unveil the effect of L-DOPA on vesicular structure. Angew. Chem., Int. Ed. 2022, 61, e202113406.
Majdi, S.; Larsson, A.; Najafinobar, N.; Borges, R.; Ewing, A. G. Extracellular ATP regulates the vesicular pore opening in chromaffin cells and increases the fraction released during individual exocytosis events. ACS Chem. Neurosci. 2019, 10, 2459–2466.
Hatamie, A.; Ren, L.; Dou, H. Q.; Gandasi, N. R.; Rorsman, P.; Ewing, A. Nanoscale amperometry reveals that only a fraction of vesicular serotonin content is released during exocytosis from beta cells. Angew. Chem., Int. Ed. 2021, 60, 7593–7596.
Zhang, X. W.; Oleinick, A.; Jiang, H.; Liao, Q. L.; Qiu, Q. F.; Svir, I.; Liu, Y. L.; Amatore, C.; Huang, W. H. Electrochemical monitoring of ROS/RNS homeostasis within individual phagolysosomes inside single macrophages. Angew. Chem., Int. Ed. 2019, 58, 7753–7756.
Hu, K. K.; Li, Y.; Rotenberg, S. A.; Amatore, C.; Mirkin, M. V. Electrochemical measurements of reactive oxygen and nitrogen species inside single phagolysosomes of living macrophages. J. Am. Chem. Soc. 2019, 141, 4564–4568.
Pan, R. R.; Wang, D. C.; Liu, K.; Chen, H. Y.; Jiang, D. C. Electrochemical molecule trap-based sensing of low-abundance enzymes in one living cell. J. Am. Chem. Soc. 2022, 144, 17558–17566.
Wang, N. N.; Wang, D. N.; Pan, R. R.; Wang, D. C.; Jiang, D. C.; Chen, H. Y. Self-referenced nanopipette for electrochemical analysis of hydrogen peroxide in the nucleus of a single living cell. Anal. Chem. 2021, 95, 10744–10749.
Dunevall, J.; Fathali, H.; Najafinobar, N.; Lovric, J.; Wigström, J.; Cans, A. S.; Ewing, A. G. Characterizing the catecholamine content of single mammalian vesicles by collision-adsorption events at an electrode. J. Am. Chem. Soc. 2015, 157, 4344–4346.
Liu, Y. Y.; Du, J. C.; Wang, M. Y.; Zhang, J.; Liu, C. L.; Li, X. C. Recent progress in quantitatively monitoring vesicular neurotransmitter release and storage with micro/nanoelectrodes. Front. Chem. 2021, 8, 591311.
Li, X. C.; Dunevall, J.; Ewing, A. G. Quantitative chemical measurements of vesicular transmitters with electrochemical cytometry. Acc. Chem. Res. 2016, 49, 2347–2354.
Zhang, X. W.; Ewing, A. G. Pore-opening dynamics of single nanometer biovesicles at an electrified interface. ACS Nano 2022, 16, 9852–9858.
Zheng, Y. N.; Nguyen, T. D. K.; Dunevall, J.; Phan, N. T. N.; Ewing, A. G. Dynamic visualization and quantification of single vesicle opening and content by coupling vesicle impact electrochemical cytometry with confocal microscopy. ACS Meas. Sci. Au 2021, 1, 131–138.
Hu, K. K.; Jia, R.; Hatamie, A.; Le Vo, K. L.; Mirkin, M. V.; Ewing, A. G. Correlating molecule count and release kinetics with vesicular size using open carbon nanopipettes. J. Am. Chem. Soc. 2020, 142, 16910–16914.
He, X. L.; Ewing, A. G. Anionic species regulate chemical storage in nanometer vesicles and amperometrically detected exocytotic dynamics. J. Am. Chem. Soc. 2022, 144, 4310–4314.
Hu, K. K.; Relton, E.; Locker, N.; Phan, N. T. N.; Ewing, A. G. Electrochemical measurements reveal reactive oxygen species in stress granules. Angew. Chem., Int. Ed. 2021, 60, 15302–15306.
He, X. L.; Ewing, A. G. Simultaneous counting of molecules in the halo and dense-core of nanovesicles by regulating dynamics of vesicle opening. Angew. Chem., Int. Ed. 2022, 61, e202116217.
Acknowledgement
We acknowledge the funding from Fundamental Research Funds for the Central Universities (No. 20720220014) and the National Natural Science Foundation of China (No. 22204134).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, C., Yang, D., Wang, Y. et al. Micro/nanoelectrode-based electrochemical methodology for single cell and organelle analysis. Nano Res. 17, 196–206 (2024). https://doi.org/10.1007/s12274-023-6210-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6210-0