Abstract
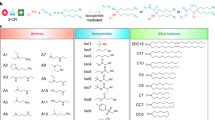
Rationally tailored lipid nanoparticles (LNPs) with efficient and tunable delivery of mRNA in vivo are crucial for mRNA vaccines. Selective expression of antigenic protein in lymphoid tissues/organs could improve the immunostimulatory efficacy and safety of LNPs-based mRNA vaccines. Inspired by the metabolic behavior that long-chain saturated fatty acids tending to enter lymphoid tissue rather than the liver, we developed fatty acid-doped LNPs capable of mediating differential protein expressions in the liver and spleen when administered intravenously. When the molar ratio of saturated fatty acid located 60%–70%, the doped LNPs achieved the spleen selective mRNA translation. The mechanism could be attributed to the different cellular uptake behaviors of saturated fatty acids in hepatocytes. Immunization with a model antigen (ovalbumin) mRNA-loaded spleen selective LNPs, we observed enhanced antigen-specific T cell immune responses, and potent immunotherapeutic and immunoprophylactic efficacy in the mouse lymphoma model. Our natural long-chain saturated fatty acids metabolic characteristics-inspired design of LNPs for spleen-selective mRNA vaccines delivery will provide references for designing mRNA vaccines with high efficacy and safety for tumor immunotherapy.

Similar content being viewed by others
References
Polack, F. P.; Thomas, S. J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J. L.; Pérez Marc, G.; Moreira, E. D.; Zerbini, C. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L. M.; Berger, H.; Petschenka, J. et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153.
Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R. A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A. N.; Omokoko, T. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112.
Miao, L.; Li, L. X.; Huang, Y. X.; Delcassian, D.; Chahal, J.; Han, J. S.; Shi, Y. H.; Sadtler, K.; Gao, W. T.; Lin, J. Q. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185.
Zhang, H. X.; You, X. R.; Wang, X. J.; Cui, L.; Wang, Z. N.; Xu, F. F.; Li, M. Y.; Yang, Z. G.; Liu, J. Y.; Huang, P. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118.
Cafri, G.; Gartner, J. J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B. C.; Parkhurst, M. R.; Yossef, R.; Lowery, F. J.; Jafferji, M. S. et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 2020, 130, 5976–5988.
Kranz, L. M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K. C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401.
Pardi, N.; Hogan, M. J.; Porter, F. W.; Weissman, D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279.
Sahin, U.; Karikö, K.; Türeci, Ö. mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780.
Kimura, S.; Khalil, I. A.; Elewa, Y. H. A.; Harashima, H. Spleen selective enhancement of transfection activities of plasmid DNA driven by octaarginine and an ionizable lipid and its implications for cancer immunization. J. Control. Release 2019, 313, 70–79.
Han, X.; Shen, S. F.; Fan, Q.; Chen, G. J.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red blood cell-derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 2019, 5, eaaw6870.
Zhang, L. X.; Jia, Y. B.; Huang, Y. R.; Liu, H. N.; Sun, X. M.; Cai, T.; Liu, R. T.; Xu, Z. P. Efficient delivery of clay-based nanovaccines to the mouse spleen promotes potent anti-tumor immunity for both prevention and treatment of lymphoma. Nano Res. 2021, 14, 1326–1334.
Gu, W. X.; An, J. N.; Li, Y. X.; Yang, Y. J.; Wang, S. M.; Shan, H.; Li, S. H.; Li, H.; Liu, G. Y.; Li, K. et al. Tuning the organ tropism of polymersome for spleen-selective nanovaccine delivery to boost cancer immunotherapy. Adv. Mater. 2023, in press, DOI: https://doi.org/10.1002/adma.202301686.
Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–755, 64–78.
Cheng, Q.; Wei, T.; Jia, Y. M.; Farbiak, L.; Zhou, K. J.; Zhang, S. Y.; Wei, Y. L.; Zhu, H.; Siegwart, D. J. Dendrimer-based lipid nanoparticles deliver therapeutic FAH mRNA to normalize liver function and extend survival in a mouse model of hepatorenal tyrosinemia type I. Adv. Mater. 2018, 30, 1805308.
Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L. T.; Dilliard, S. A.; Siegwart, D. J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320.
Dammes, N.; Goldsmith, M.; Ramishetti, S.; Dearling, J. L. J.; Veiga, N.; Packard, A. B.; Peer, D. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 2021, 16, 1030–1038.
Qiu, M.; Tang, Y.; Chen, J. J.; Muriph, R.; Ye, Z. F.; Huang, C. F.; Evans, J.; Henske, E. P.; Xu, Q. B. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116271119.
Sago, C. D.; Lokugamage, M. P.; Loughrey, D.; Lindsay, K. E.; Hincapie, R.; Krupczak, B. R.; Kalathoor, S.; Sato, M.; Echeverri, E. S.; Fitzgerald, J. P. et al. Augmented lipid-nanoparticle-mediated in vivo genome editing in the lungs and spleen by disrupting Cas9 activity in the liver. Nat. Biomed. Eng. 2022, 6, 157–167.
Zhao, X. W.; Chen, J. J.; Qiu, M.; Li, Y. M.; Glass, Z.; Xu, Q. B. Imidazole-based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem., Int. Ed. 2020, 59, 20083–20089.
Liu, S.; Wang, X.; Yu, X. L.; Cheng, Q.; Johnson, L. T.; Chatterjee, S.; Zhang, D.; Lee, S. M.; Sun, Y. H.; Lin, T. C. et al. Zwitterionic phospholipidation of cationic polymers facilitates systemic mRNA delivery to spleen and lymph nodes. J. Am. Chem. Soc. 2021, 143, 21321–21330.
Liu, S.; Cheng, Q.; Wei, T.; Yu, X. L.; Johnson, L. T.; Farbiak, L.; Siegwart, D. J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021, 20, 701–710.
Hagenfeldt, L.; Wahren, J.; Pernow, B.; Räf, L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J. Clin. Invest. 1972, 51, 2324–2330.
Soler-Argilaga, C.; Infante, R.; Polonovski, J. Influence of chain length and degree of unsaturation on plasma free fatty acid uptake by the perfused rat liver. Biochim. Biophys. Acta Lipids Lipid Metab. 1973, 326, 167–173.
Emmison, N.; Agius, L. Fatty acid uptake and metabolism to ketone bodies and triacylglycerol in rat and human hepatocyte cultures is dependent on chain-length and degree of saturation effects of carnitine and glucagon. FEBS Lett. 1988, 236, 83–88.
Kuhn, J.; Lin, Y.; Krhac Levacic, A.; Al Danaf, N.; Peng, L.; Höhn, M.; Lamb, D. C.; Wagner, E.; Lächelt, U. Delivery of Cas9/sgRNA ribonucleoprotein complexes via hydroxystearyl oligoamino amides. Bioconjug. Chem. 2020, 31, 729–742.
Lee, S. M.; Cheng, Q.; Yu, X. L.; Liu, S.; Johnson, L. T.; Siegwart, D. J. A systematic study of unsaturation in lipid nanoparticles leads to improved mRNA transfection in vivo. Angew. Chem., Int. Ed. 2021, 60, 5848–5853.
Wei, T.; Cheng, Q.; Min, Y. L.; Olson, E. N.; Siegwart, D. J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020, 11, 3232.
Shobaki, N.; Sato, Y.; Harashima, H. Mixing lipids to manipulate the ionization status of lipid nanoparticles for specific tissue targeting. Int. J. Nanomed. 2018, 13, 8395–8410.
Fenton, O. S.; Kauffman, K. J.; Kaczmarek, J. C.; McClellan, R. L.; Jhunjhunwala, S.; Tibbitt, M. W.; Zeng, M. D.; Appel, E. A.; Dorkin, J. R.; Mir, F. F. et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 2017, 29, 1606944.
Luozhong, S.; Yuan, Z. F.; Sarmiento, T.; Chen, Y.; Gu, W. C.; McCurdy, C.; Gao, W. T.; Li, R. X.; Wilkens, S.; Jiang, S. Y. Phosphatidylserine lipid nanoparticles promote systemic RNA delivery to secondary lymphoid organs. Nano Lett. 2022, 22, 8304–8311.
Fröhlich, T.; Edinger, D.; Kläger, R.; Troiber, C.; Salcher, E.; Badgujar, N.; Martin, I.; Schaffert, D.; Cengizeroglu, A.; Hadwiger, P. et al. Structure-activity relationships of siRNA carriers based on sequence-defined oligo (ethane amino) amides. J. Control. Release 2012, 160, 532–541.
Sedic, M.; Senn, J. J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S. J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the sprague-dawley rat and cynomolgus monkey. Vet. Pathol. 2018, 55, 341–354.
Huang, X. G.; Kong, N.; Zhang, X. C.; Cao, Y. H.; Langer, R.; Tao, W. The landscape of mRNA nanomedicine. Nat. Med. 2022, 28, 2273–2287.
Kulkarni, J. A.; Darjuan, M. M.; Mercer, J. E.; Chen, S.; Van Der Meel, R.; Thewalt, J. L.; Tam, Y. Y. C.; Cullis, P. R. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano 2018, 12, 4787–4795.
Godoy, P.; Hewitt, N. J.; Albrecht, U.; Andersen, M. E.; Ansari, N.; Bhattacharya, S.; Bode, J. G.; Bolleyn, J.; Borner, C.; Böttger, J. et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530.
Sato, Y.; Hatakeyama, H.; Hyodo, M.; Harashima, H. Relationship between the physicochemical properties of lipid nanoparticles and the quality of siRNA delivery to liver cells. Mol. Ther. 2016, 24, 788–795.
Dilliard, S. A.; Cheng, Q.; Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118.
Xiong, H.; Liu, S.; Wei, T.; Cheng, Q.; Siegwart, D. J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J. Control. Release 2020, 325, 198–205.
Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith III, J. P.; Hou, S. G.; Esposito, A. A.; Ketova, T.; Welsher, K. et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983.
Pan, L. Z.; Zhang, L. J.; Deng, W. J.; Lou, J.; Gao, X. K.; Lou, X. H.; Liu, Y. Y.; Yao, X. H.; Sheng, Y. Q.; Yan, Y. et al. Spleen-selective co-delivery of mRNA and TLR4 agonist-loaded LNPs for synergistic immunostimulation and Th1 immune responses. J. Control. Release 2023, 357, 133–148.
Fan, Y. N.; Li, M.; Luo, Y. L.; Chen, Q.; Wang, L.; Zhang, H. B.; Shen, S.; Gu, Z.; Wang, J. Cationic lipid-assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater. Sci. 2018, 6, 3009–3018.
Van Der Jeught, K.; De Koker, S.; Bialkowski, L.; Heirman, C.; Tjok Joe, P.; Perche, F.; Maenhout, S.; Bevers, S.; Broos, K.; Deswarte, K. et al. Dendritic Cell Targeting mRNA Lipopolyplexes Combine Strong Antitumor T-Cell Immunity with Improved Inflammatory Safety. ACS Nano 2018, 12, 9815–9829.
Wculek, S. K.; Cueto, F. J.; Mujal, A. M.; Melero, I.; Krummel, M. F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24.
Baharom, F.; Ramirez-Valdez, R. A.; Khalilnezhad, A.; Khalilnezhad, S.; Dillon, M.; Hermans, D.; Fussell, S.; Tobin, K. K. S.; Dutertre, C. A.; Lynn, G. M. et al. Systemic vaccination induces CD8+ T cells and remodels the tumor microenvironment. Cell 2022, 185, 4317–4332.e15.
Broos, K.; Van Der Jeught, K.; Puttemans, J.; Goyvaerts, C.; Heirman, C.; Dewitte, H.; Verbeke, R.; Lentacker, I.; Thielemans, K.; Breckpot, K. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol. Ther. Nucleic Acids 2016, 5, e326.
Le Moignic, A.; Malard, V.; Benvegnu, T.; Lemiègre, L.; Berchel, M.; Jaffrès, P. A.; Baillou, C.; Delost, M.; Macedo, R.; Rochefort, J. et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Control. Release 2018, 278, 110–121.
Zhang, L. X.; Sun, X. M.; Jia, Y. B.; Liu, X. G.; Dong, M. D.; Xu, Z. P.; Liu, R. T. Nanovaccine’s rapid induction of anti-tumor immunity significantly improves malignant cancer immunotherapy. Nano Today 2020, 35, 100923.
Oberli, M. A.; Reichmuth, A. M.; Dorkin, J. R.; Mitchell, M. J.; Fenton, O. S.; Jaklenec, A.; Anderson, D. G.; Langer, R.; Blankschtein, D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017, 17, 1326–1335.
Grunwitz, C.; Salomon, N.; Vascotto, F.; Selmi, A.; Bukur, T.; Diken, M.; Kreiter, S.; Türeci, Ö.; Sahin, U. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology 2019, 8, e1629259.
Reinhard, K.; Rengstl, B.; Oehm, P.; Michel, K.; Billmeier, A.; Hayduk, N.; Klein, O.; Kuna, K.; Ouchan, Y.; Wöll, S. et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 2020, 367, 446–453.
Acknowledgements
This study was financial supported by the National Key Research and Development Program of China (No. 2021YFA1201102), Henan Medical Science and Technology Joint Building Program (No. SBGJ202102132), Henan Province Youth Talent Promoting Project (No. 2022HYTP047), the National Natural Science Foundation of China (Nos. 82003255, 82101385 and 82073231), Key Research and Development Project of Henan Province (No. 232102311224) and First-Class Clinical Medicine Discipline Construction Talents Cultivation Project of Zhengzhou University. Thanks are given to the Home for Researchers (https://www.home-for-researchers.com) for drawing the scheme.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_6111_MOESM1_ESM.pdf
Natural long-chain saturated fatty acids doped LNPs enabling spleen selective mRNA translation and potent cancer immunotherapy
Rights and permissions
About this article
Cite this article
Wang, F., Zhang, M., Tian, M. et al. Natural long-chain saturated fatty acids doped LNPs enabling spleen selective mRNA translation and potent cancer immunotherapy. Nano Res. 17, 1804–1817 (2024). https://doi.org/10.1007/s12274-023-6111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6111-2