Abstract
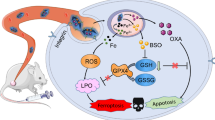
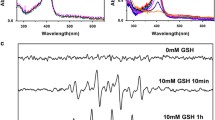
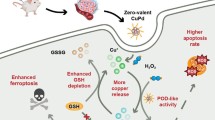
Artemisinin and its derivatives have emerged as promising therapeutic agents for cancer therapy by endogenous iron-mediated generation of free radicals. However, the enhanced antioxidant defense systems in cancer cells provide them with resistance to oxidative damage, greatly antagonizing the therapeutic efficacy that relies on inducing oxidative stress. Herein, a metal-organic framework (MOF)-based nanoplatform (CMD) is constructed to disrupt the cellular redox homeostasis and selectively potentiate the cytotoxicity of dihydroartemisinin for cancer therapy. In cancer cells, the copper(II) sites in the MOF nanocarrier of CMD can efficiently weaken the cellular antioxidant capacity by depleting the overexpressed glutathione, simultaneously leading to the decomposition of the framework structure and the release of the encapsulated dihydroartemisinin. As a result, the damaged antioxidant defense system of cancer cells reduces its effect on oxidative stress alleviation and strengthens the therapeutic efficacy of dihydroartemisinin. On contrast, the low concentration of cellular glutathione in normal cells protects them from dihydroartemisinin-induced cytotoxicity by decelerating the drug release. In vivo results demonstrate that CMD could completely suppress the tumor growth in mice and show no evidence of toxicity, providing an effective strategy for the practical usage of dihydroartemisinin in cancer therapy.

Similar content being viewed by others
References
Chaturvedi, D.; Goswami, A.; Pratim Saikia, P.; Barua, N. C.; Rao, P. G. Artemisinin and its derivatives: A novel class of anti-malarial and anti-cancer agents. Chem. Soc. Rev. 2010, 39, 435–454.
Wan, X. Y.; Zhong, H.; Pan, W.; Li, Y. H.; Chen, Y. Y.; Li, N.; Tang, B. Programmed release of dihydroartemisinin for synergistic cancer therapy using a CaCO3 mineralized metal-organic framework. Angew. Chem., Int. Ed. 2019, 58, 14134–14139.
Chen, G. Q.; Benthani, F. A.; Wu, J.; Liang, D. G.; Bian, Z. X.; Jiang, X. J. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020, 27, 242–254.
Gorrini, C.; Harris, I. S.; Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947.
Wang, H.; Liu, X. C.; Yan, X. Y.; Fan, J. W.; Li, D. W.; Ren, J. S.; Qu, X. G. A MXene-derived redox homeostasis regulator perturbs the Nrf2 antioxidant program for reinforced sonodynamic therapy. Chem. Sci. 2022, 13, 6704–6714.
Chen, X.; Kang, R.; Kroemer, G.; Tang, D. L. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296.
Xiong, Y. X.; Xiao, C.; Li, Z. F.; Yang, X. L. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem. Soc. Rev. 2021, 50, 6013–6041.
Fan, H. H.; Yan, G. B.; Zhao, Z. L.; Hu, X. X.; Zhang, W. H.; Liu, H.; Fu, X. Y.; Fu, T.; Zhang, X. B.; Tan, W. H. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew. Chem., Int. Ed. 2016, 55, 5477–5482.
Ju, E. G.; Dong, K.; Chen, Z. W.; Liu, Z.; Liu, C. Q.; Huang, Y. Y.; Wang, Z. Z.; Pu, F.; Ren, J. S.; Qu, X. G. Copper(II)-graphitic carbon nitride triggered synergy: Improved ROS generation and reduced glutathione levels for enhanced photodynamic therapy. Angew. Chem., Int. Ed. 2016, 55, 11467–11471.
Zhang, W.; Lu, J.; Gao, X. N.; Li, P.; Zhang, W.; Ma, Y.; Wang, H.; Tang, B. Enhanced photodynamic therapy by reduced levels of intracellular glutathione obtained by employing a nano-MOF with CuII as the active center. Angew. Chem., Int. Ed. 2018, 57, 4891–4896.
Gong, F.; Cheng, L.; Yang, N. L.; Betzer, O.; Feng, L. Z.; Zhou, Q.; Li, Y. G.; Chen, R. H.; Popovtzer, R.; Liu, Z. Ultrasmall oxygen-deficient bimetallic oxide MnWOx nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv. Mater. 2019, 31, 1900730.
Gong, N. Q.; Ma, X. W.; Ye, X. X.; Zhou, Q. F.; Chen, X. A.; Tan, X. L.; Yao, S. K.; Huo, S. D.; Zhang, T. B.; Chen, S. Z. et al. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat. Nanotechnol. 2019, 14, 379–387.
Zhao, B.; Wang, Y. S.; Yao, X. X.; Chen, D. Y.; Fan, M. J.; Jin, Z. K.; He, Q. J. Photocatalysis-mediated drug-free sustainable cancer therapy using nanocatalyst. Nat. Commun. 2021, 12, 1345.
Liu, C. H.; Cao, Y.; Cheng, Y. R.; Wang, D. D.; Xu, T. L.; Su, L.; Zhang, X. J.; Dong, H. F. An open source and reduce expenditure ROS generation strategy for chemodynamic/photodynamic synergistic therapy. Nat. Commun. 2020, 11, 1735.
Niu, B. Y.; Liao, K. X.; Zhou, Y. X.; Wen, T.; Quan, G. L.; Pan, X.; Wu, C. B. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110.
Liu, L. J.; Wei, Y. C.; Zhai, S. D.; Chen, Q.; Xing, D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials 2015, 62, 35–46.
Dong, L.; Wang, C.; Zhen, W. Y.; Jia, X. D.; An, S. J.; Xu, Z. A.; Zhang, W.; Jiang, X. E. Biodegradable iron-coordinated hollow polydopamine nanospheres for dihydroartemisinin delivery and selectively enhanced therapy in tumor cells. J. Mater. Chem. B 2019, 7, 6172–6180.
He, C. B.; Liu, D. M.; Lin, W. B. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108.
Lu, K. D.; Aung, T.; Guo, N. N.; Weichselbaum, R.; Lin, W. B. Nanoscale metal-organic frameworks for therapeutic, imaging, and sensing applications. Adv. Mater. 2018, 30, 1707634.
Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater. 2018, 30, 1707365.
Wu, M. X.; Yang, Y. W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134.
Liu, J. T.; Huang, J.; Zhang, L.; Lei, J. P. Multifunctional metal-organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 1188–1218.
Zheng, Q. L.; Liu, X. M.; Zheng, Y. F.; Yeung, K. W. K.; Cui, Z. D.; Liang, Y. Q.; Li, Z. Y.; Zhu, S. L.; Wang, X. B.; Wu, S. L. The recent progress on metal-organic frameworks for phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125.
Gao, P.; Chen, Y. Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor agents based on metal-organic frameworks. Angew. Chem., Int. Ed. 2021, 60, 16763–16776.
Wang, Y. B.; Xu, S. D.; Shi, L. L.; Teh, C.; Qi, G. B.; Liu, B. Cancer-cell-activated in situ synthesis of mitochondria-targeting AIE photosensitizer for precise photodynamic therapy. Angew. Chem., Int. Ed. 2021, 60, 14945–14953.
Liu, Q.; Jin, L. N.; Sun, W. Y. Facile fabrication and adsorption property of a nano/microporous coordination polymer with controllable size and morphology. Chem. Commun. 2012, 48, 8814–8816.
Wang, Y. B.; Wu, W. B.; Liu, J. J.; Manghnani, P. N.; Hu, F.; Ma, D.; Teh, C.; Wang, B.; Liu, B. Cancer-cell-activated photodynamic therapy assisted by Cu(II)-based metal-organic framework. ACS Nano 2019, 13, 6879–6890.
Acknowledgements
This work was supported by the Program of Science and Technology Development Plan of Jilin Province of China (No. 20200201099JC), and the National Natural Science Foundation of China (Nos. 21871249 and 22105197).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_5385_MOESM1_ESM.pdf
A metal-organic framework-based redox homeostasis disruptor selectively potentiate the cytotoxicity of dihydroartemisinin for cancer therapy
Rights and permissions
About this article
Cite this article
Fan, J., Liu, X., Wang, Q. et al. A metal-organic framework-based redox homeostasis disruptor selectively potentiate the cytotoxicity of dihydroartemisinin for cancer therapy. Nano Res. 16, 7489–7495 (2023). https://doi.org/10.1007/s12274-023-5385-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5385-8