Abstract
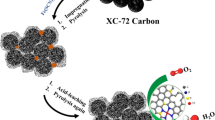
The synergistic catalysis of heterojunction electrocatalysts for the multi-step process in hydrogen evolution reaction (HER) is a promising approach to enhance the kinetics of alkaline HER. Herein, we proposed a strategy to form nanoscale Ni/NiO heterojunction porous graphitic carbon composites (Ni/NiO-PGC) by reduction-pyrolysis of the preformed Ni-metal-organic framework (MOF) under H2/N2 atmosphere. Benefiting from low electron transfer resistance, increased number of active sites, and unique hierarchical micro-mesoporous structure, the optimized Ni/NiO-PGC10−1−400 exhibited excellent electrocatalytic performance and robust stability for alkaline HER (η10 = 30 mV, 65 h). Density functional theory (DFT) studies revealed that the redistribution of electrons at the Ni/NiO interface enables the NiO phase to easily initiate the dissociation of alkaline H2O, and shifts down the d-band center of Ni and optimizes the H* adsorption-desorption process of Ni, thereby leading to extremely high HER activity. This work contributes to a further understanding of the synergistic promotion of the multi-step HER processes by heterojunction electrocatalysts.

Similar content being viewed by others
References
van Troostwijk, A. P.; Deiman, J. R. Sur une manière de decomposer l’eau en air inflammable & en air vital. Obs. Phys. 1789, 35, 369–378.
Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques and Applications, 2nd ed.; Wiley-VCH: Weinheim, 2018.
Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181.
Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S. H.; Shao, Z. P.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196.
Zeng, H. B.; Chen, S. Q.; Jin, Y. Q.; Li, J. W.; Song, J. D.; Le, Z. C.; Liang, G. F.; Zhang, H.; Xie, F. Y.; Chen, J. et al. Electron density modulation of metallic MoO2 by Ni doping to produce excellent hydrogen evolution and oxidation activities in acid. ACS Energy Lett. 2020, 5, 1908–1915.
Chen, L.; Dong, X. L.; Wang, Y. G.; Xia, Y. Y. Separating hydrogen and oxygen evolution in alkaline water electrolysis using nickel hydroxide. Nat. Commun. 2016, 7, 11741.
Norskov, J. K.; Christensen, C. H. Toward efficient hydrogen production at surfaces. Science 2006, 312, 1322–1323.
Bai, S.; Wang, C. M.; Deng, M. S.; Gong, M.; Bai, Y.; Jiang, J.; Xiong, Y. J. Surface polarization matters: Enhancing the hydrogen-evolution reaction by shrinking Pt shells in Pt-Pd-graphene stack structures. Angew. Chem., Int. Ed. 2014, 53, 12120–12124.
Wei, J. M.; Zhou, M.; Long, A. C.; Xue, Y. M.; Liao, H. B.; Wei, C.; Xu, Z. J. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nano-Micro Lett. 2018, 10, 75.
Du, Y. X.; Sheng, H. T.; Astruc, D.; Zhu, M. Z. Atomically precise noble metal nanoclusters as efficient catalysts: A bridge between structure and properties. Chem. Rev. 2020, 120, 526–622.
Liu, Y. K.; Hu, B.; Wu, S. D.; Wang, M. H.; Zhang, Z. H.; Cui, B. B.; He, L. H.; Du, M. Hierarchical nanocomposite electrocatalyst of bimetallic zeolitic imidazolate framework and MoS2 sheets for non-Pt methanol oxidation and water splitting. Appl. Catal. B: Environ. 2019, 258, 117970.
Liu, D. B.; Li, X. Y.; Chen, S. M.; Yan, H.; Wang, C. D.; Wu, C. Q.; Haleem, Y. A.; Duan, S.; Lu, J. L.; Ge, B. H. et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518.
Nairan, A.; Liang, C. W.; Chiang, S. W.; Wu, Y.; Zou, P. C.; Khan, U.; Liu, W. D.; Kang, F. Y.; Guo, S. J.; Wu, J. B. et al. Proton selective adsorption on Pt-Ni nano-thorn array electrodes for superior hydrogen evolution activity. Energy Environ. Sci. 2021, 14, 1594–1601.
Zhang, G. F.; Wang, A. H.; Niu, L. W.; Gao, W.; Hu, W.; Liu, Z. X.; Wang, R. M.; Chen, J. B. Interfacial engineering to construct antioxidative Pd4S/Pd3P0.95 heterostructure for robust hydrogen production at high current density. Adv. Energy Mater. 2022, 12, 2103511.
Fu, H. Q.; Zhou, M.; Liu, P. F.; Liu, P. R.; Yin, H. J.; Sun, K. Z.; Yang, H. G.; Al-Mamun, M.; Hu, P. J.; Wang, H. F. et al. Hydrogen spillover-bridged Volmer/Tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential. J. Am. Chem. Soc. 2022, 144, 6028–6039.
Lei, C. J.; Wang, Y.; Hou, Y.; Liu, P.; Yang, J.; Zhang, T.; Zhuang, X. D.; Chen, M. W.; Yang, B.; Lei, L. C. et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni−Nx species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics. Energy Environ. Sci. 2019, 12, 149–156.
Pattengale, B.; Huang, Y. C.; Yan, X. X.; Yang, S. Z.; Younan, S.; Hu, W. H.; Li, Z. D.; Lee, S.; Pan, X. Q.; Gu, J. et al. Dynamic evolution and reversibility of single-atom Ni(II) active site in 1T-MoS2 electrocatalysts for hydrogen evolution. Nat. Commun. 2020, 11, 4114.
Kim, J.; Jung, H.; Jung, S. M.; Hwang, J.; Kim, D. Y.; Lee, N.; Kim, K. S.; Kwon, H.; Kim, Y. T.; Han, J. W. et al. Tailoring binding abilities by incorporating oxophilic transition metals on 3D nanostructured Ni arrays for accelerated alkaline hydrogen evolution reaction. J. Am. Chem. Soc. 2021, 143, 1399–1408.
Zhang, J. Y.; Liang, J. Y.; Mei, B. B.; Lan, K.; Zu, L. H.; Zhao, T. C.; Ma, Y. Z.; Chen, Y.; Lv, Z. R.; Yang, Y. et al. Synthesis of Ni/NiO@MoO3−x composite nanoarrays for high current density hydrogen evolution reaction. Adv. Energy Mater. 2022, 12, 2200001.
Dinh, C. T.; Jain, A.; de Arquer, F. P. G.; De Luna, P.; Li, J.; Wang, N.; Zheng, X. L.; Cai, J.; Gregory, B. Z.; Voznyy, O. et al. Multi-site electrocatalysts for hydrogen evolution in neutral media by destabilization of water molecules. Nat. Energy 2019, 4, 107–114.
Jin, H. Y.; Wang, J.; Su, D. F.; Wei, Z. Z.; Pang, Z. F.; Wang, Y. In situ cobalt-cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694.
Peng, L. S.; Zheng, X. Q.; Li, L.; Zhang, L.; Yang, N.; Xiong, K.; Chen, H. M.; Li, J.; Wei, Z. D. Chimney effect of the interface in metal oxide/metal composite catalysts on the hydrogen evolution reaction. Appl. Catal. B: Environ. 2019, 245, 122–129.
Zhu, Y. L.; Lin, Q.; Zhong, Y. J.; Tahini, H. A.; Shao, Z. P.; Wang, H. T. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392.
Liu, Y.; Liu, X. H.; Wang, X. S.; Ning, H.; Yang, T.; Yu, J. M.; Kumar, A.; Luo, Y. G.; Wang, H. D.; Wang, L. L. et al. Unraveling the synergy of chemical hydroxylation and the physical heterointerface upon improving the hydrogen evolution kinetics. ACS Nano 2021, 15, 15017–15026.
Hu, K. L.; Ohto, T.; Chen, L. H.; Han, J. H.; Wakisaka, M.; Nagata, Y.; Fujita, J. I.; Ito, Y. Graphene layer encapsulation of non-noble metal nanoparticles as acid-stable hydrogen evolution catalysts. ACS Energy Lett. 2018, 3, 1539–1544.
Li, X. P.; Wang, Y.; Wang, J. J.; Da, Y. M.; Zhang, J. F.; Li, L. L.; Zhong, C.; Deng, Y. D.; Han, X. P.; Hu, W. B. Sequential electrodeposition of bifunctional catalytically active structures in MoO3/Ni-NiO composite electrocatalysts for selective hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 2003414.
He, H. Z.; Zhang, Y.; Zhang, W. Q.; Li, Y. Y.; Wang, Y.; Wang, P.; Hu, D. M. Dual metal-loaded porous carbon materials derived from silk fibroin as bifunctional electrocatalysts for hydrogen evolution reaction and oxygen evolution reaction. ACS Appl. Mater. Interfaces 2021, 13, 30678–30692.
Yang, C. C.; Zai, S. F.; Zhou, Y. T.; Du, L.; Jiang, Q. Fe3C−Co nanoparticles encapsulated in a hierarchical structure of N-doped carbon as a multifunctional electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949.
Li, B. L.; Li, Z. S.; Pang, Q.; Zhang, J. Z. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045.
Cao, Y. Y.; Lu, Y. D.; Ang, E. H.; Geng, H. B.; Cao, X. Q.; Zheng, J. W.; Gu, H. W. MOF-derived uniform Ni nanoparticles encapsulated in carbon nanotubes grafted on rGO nanosheets as bifunctional materials for lithium-ion batteries and hydrogen evolution reaction. Nanoscale 2019, 11, 15112–15119.
Wang, T.; Guo, Y. R.; Zhou, Z. X.; Chang, X. H.; Zheng, J.; Li, X. G. Ni−Mo nanocatalysts on N-doped graphite nanotubes for highly efficient electrochemical hydrogen evolution in acid. ACS Nano 2016, 10, 10397–10403.
Yang, Z. K.; Zhao, C. M.; Qu, Y. T.; Zhou, H.; Zhou, F. Y.; Wang, J.; Wu, Y. E.; Li, Y. D. Trifunctional self-supporting cobalt-embedded carbon nanotube films for ORR, OER, and HER triggered by solid diffusion from bulk metal. Adv. Mater. 2019, 31, 1808043.
Yan, L. T.; Xu, Y. L.; Chen, P.; Zhang, S.; Jiang, H. M.; Yang, L. Z.; Wang, Y.; Zhang, L.; Shen, J. X.; Zhao, X. B. et al. A freestanding 3D heterostructure film stitched by MOF-derived carbon nanotube microsphere superstructure and reduced graphene oxide sheets: A superior multifunctional electrode for overall water splitting and Zn-air batteries. Adv. Mater. 2020, 32, 2003313.
Liu, X. E.; Liu, W.; Ko, M.; Park, M.; Kim, M. G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G. et al. Metal (Ni, Co)-metal oxides/graphene nanocomposites as multifunctional electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808.
Gong, M.; Zhou, W.; Tsai, M. C.; Zhou, J. G.; Guan, M. Y.; Lin, M. C.; Zhang, B.; Hu, Y. F.; Wang, D. Y.; Yang, J. et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695.
Mateo, D.; Albero, J.; García, H. Graphene supported NiO/Ni nanoparticles as efficient photocatalyst for gas phase CO2 reduction with hydrogen. Appl. Catal. B: Environ. 2018, 224, 563–571.
Bian, Y. R.; Wang, H.; Gao, Z.; Hu, J. T.; Liu, D.; Dai, L. M. A facile approach to high-performance trifunctional electrocatalysts by substrate-enhanced electroless deposition of Pt/NiO/Ni on carbon nanotubes. Nanoscale 2020, 12, 14615–14625.
Lu, X. F.; Xia, B. Y.; Zang, S. Q.; Lou, X. W. Metal-organic frameworks based electrocatalysts for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2020, 59, 4634–4650.
Jiao, Y.; Hong, W. Z.; Li, P. Y.; Wang, L. X.; Chen, G. Metal-organic framework derived Ni/NiO micro-particles with subtle lattice distortions for high-performance electrocatalyst and supercapacitor. Appl. Catal. B: Environ. 2019, 244, 732–739.
Li, Z.; Song, M.; Zhu, W. Y.; Zhuang, W. C.; Du, X. H.; Tian, L. MOF-derived hollow heterostructures for advanced electrocatalysis. Coord. Chem. Rev. 2021, 439, 213946.
Carton, A.; Mesbah, A.; Mazet, T.; Porcher, F.; François, M. Ab initio crystal structure of nickel(II) hydroxy-terephthalate by synchrotron powder diffraction and magnetic study. Solid State Sci. 2007, 9, 465–471.
Huang, J. Z.; Han, J. C.; Wu, T.; Feng, K.; Yao, T.; Wang, X. J.; Liu, S. W.; Zhong, J.; Zhang, Z. H.; Zhang, Y. M. et al. Boosting hydrogen transfer during Volmer reaction at oxides/metal nanocomposites for efficient alkaline hydrogen evolution. ACS Energy Lett. 2019, 4, 3002–3010.
Zhao, L.; Zhang, Y.; Zhao, Z. L.; Zhang, Q. H.; Huang, L. B.; Gu, L.; Lu, G.; Hu, J. S.; Wan, L. J. Steering elementary steps towards efficient alkaline hydrogen evolution via size-dependent Ni/NiO nanoscale heterosurfaces. Natl. Sci. Rev. 2020, 7, 27–36.
Gu, C. J.; Zhou, G. Y.; Yang, J.; Pang, H.; Zhang, M. Y.; Zhao, Q.; Gu, X. F.; Tian, S.; Zhang, J. B.; Xu, L. et al. NiS/MoS2 Mott-Schottky heterojunction-induced local charge redistribution for high-efficiency urea-assisted energy-saving hydrogen production. Chem. Eng. J. 2022, 443, 136321.
Yang, Y.; Lun, Z. Y.; Xia, G. L.; Zheng, F. C.; He, M. N.; Chen, Q. W. Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571.
Zhu, Y.; Zhang, J. H.; Qian, Q. Z.; Li, Y. P.; Li, Z. Y.; Liu, Y.; Xiao, C.; Zhang, G. Q.; Xie, Y. Dual nanoislands on Ni/C hybrid nanosheet activate superior hydrazine oxidation-assisted high-efficiency H2 production. Angew. Chem., Int. Ed. 2022, 61, e202113082.
Song, S.; Yao, S. K.; Cao, J. H.; Di, L.; Wu, G. J.; Guan, N. J.; Li, L. D. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B: Environ. 2017, 217, 115–124.
Li, N.; Li, Y.; Li, Q.; Zhao, Y.; Liu, C. S.; Pang, H. NiO nanoparticles decorated hexagonal nickel-based metal-organic framework: Self-template synthesis and its application in electrochemical energy storage. J. Colloid Interface Sci. 2021, 581, 709–718.
Yang, Y.; Sun, X. D.; Han, G. Q.; Liu, X.; Zhang, X. Y.; Sun, Y. F.; Zhang, M.; Cao, Z.; Sun, Y. J. Enhanced electrocatalytic hydrogen oxidation on Ni/NiO/C derived from a nickel-based metal-organic framework. Angew. Chem., Int. Ed. 2019, 58, 10644–10649.
Zhang, Y. F.; Su, Q.; Xu, W. J.; Cao, G. Z.; Wang, Y. P.; Pan, A. Q.; Liang, S. Q. A confined replacement synthesis of bismuth nanodots in MOF derived carbon arrays as binder-free anodes for sodium-ion batteries. Adv. Sci. 2019, 6, 1900162.
Wang, W. Z.; Liu, Y. K.; Xu, C. K.; Zheng, C. L.; Wang, G. H. Synthesis of NiO nanorods by a novel simple precursor thermal decomposition approach. Chem. Phys. Lett. 2002, 362, 119–122.
Mir, R. A.; Pandey, O. P. Influence of graphitic/amorphous coated carbon on HER activity of low temperature synthesized β-Mo2C@C nanocomposites. Chem. Eng. J. 2018, 348, 1037–1048.
Yi, X. R.; He, X. B.; Yin, F. X.; Li, G. R.; Li, Z. C. Surface strain engineered Ni−NiO for boosting hydrogen evolution reaction in alkaline media. Electrochim. Acta 2021, 391, 138985.
Campos-Roldán, C. A.; Calvillo, L.; Boaro, M.; de Guadalupe González-Huerta, R.; Granozzi, G.; Alonso-Vante, N. NiO−Ni/CNT as an efficient hydrogen electrode catalyst for a unitized regenerative alkaline microfluidic cell. ACS Appl. Energy Mater. 2020, 3, 4746–4755.
Anisimov, V. I.; Solovyev, I. V.; Korotin, M. A.; Czyżyk, M. T.; Sawatzky, G. A. Density-functional theory and NiO photoemission spectra. Phys. Rev. B 1993, 48, 16929–16934.
Sun, H.; Lian, Y. B.; Yang, C.; Xiong, L. K.; Qi, P. W.; Mu, Q. Q.; Zhao, X. H.; Guo, J.; Deng, Z.; Peng, Y. A hierarchical nickel-carbon structure templated by metal-organic frameworks for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 2363–2371.
Ma, X. Q.; Tang, K. L.; Yang, M. Y.; Shi, W. B.; Zhao, W. X. Metal-organic framework-derived yolk-shell hollow Ni/NiO@C microspheres for bifunctional non-enzymatic glucose and hydrogen peroxide biosensors. J. Mater. Sci. 2021, 56, 442–456.
Wang, J. M.; Zhao, Z.; Shen, C.; Liu, H. P.; Pang, X. Y.; Gao, M. Q.; Mu, J.; Cao, F.; Li, G. Q. Ni/NiO heterostructures encapsulated in oxygen-doped graphene as multifunctional electrocatalysts for the HER, UOR and HMF oxidation reaction. Catal. Sci. Technol. 2021, 11, 2480–2490.
Peng, Y. W.; Zhao, M. T.; Chen, B.; Zhang, Z. C.; Huang, Y.; Dai, F. N.; Lai, Z. C.; Cui, X. Y.; Tan, C. L.; Zhang, H. Hybridization of MOFs and COFs: A new strategy for construction of MOF@COF core-shell hybrid materials. Adv. Mater. 2018, 30, 1705454.
Zhou, S. Y.; Wang, S.; Zhou, S. J.; Xu, H. B.; Zhao, J. P.; Wang, J.; Li, Y. An electrochromic supercapacitor based on an MOF derived hierarchical-porous NiO film. Nanoscale 2020, 12, 8934–8941.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22271203, 21773163, and 22001021), the State Key Laboratory of Organometallic Chemistry of Shanghai Institute of Organic Chemistry (No. KF2021005), the Natural Science Foundation of Jiangsu Province (No. BK20201048), the Natural Science Research Project of Higher Education Institutions in Jiangsu Province (No. 20KJB150008), the Collaborative Innovation Center of Suzhou Nano Science and Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Project of Scientific and Technologic Infrastructure of Suzhou (No. SZS201905). We are grateful to the useful comments and suggestions from the editor and the reviewers.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_5194_MOESM1_ESM.pdf
Accelerating water dissociation at carbon supported nanoscale Ni/NiO heterojunction electrocatalysts for high-efficiency alkaline hydrogen evolution
Rights and permissions
About this article
Cite this article
Li, C., Xue, JY., Zhang, W. et al. Accelerating water dissociation at carbon supported nanoscale Ni/NiO heterojunction electrocatalysts for high-efficiency alkaline hydrogen evolution. Nano Res. 16, 4742–4750 (2023). https://doi.org/10.1007/s12274-022-5194-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5194-5