Abstract
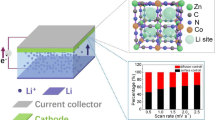
Mn-based Prussian blue analogues (Mn-PBAs), featuring a three-dimensional (3D) metal-organic framework and multiple redox couples, have gained wide interests in Zn-ion batteries (ZIBs). However, owing to the Jahn-Teller distortion and disproportionation reaction of Mn3+, these materials suffer from poor electrochemical performances and inferior structural stability. Herein, we prepare a typical high-entropy Prussian blue analogue (HE-PBA) with increased configuration entropy through integrating five transition metal elements of Mn, Co, Ni, Fe and Cu into the nitrogen-coordinated -M- lattice sites. Consequently, the HE-PBA presents enhanced uptake of Zn2+ with 80 mAh·g−1 compared to those medium-entropy PBAs, low-entropy PBAs and conventional PBAs, which can be assigned to “cocktail” effect of multiple transition metal active redox couples. Furthermore, a phase transition process from monoclinic phase to rhombohedral phase occurs in HE-PBA cathode, resulting in a stable structure of MN6 (M = Mn, Co, Fe, Ni, Cu) and ZnN4 co-linked to FeC6 through the cyanide ligands. Additionally, the advantages of entropy-driven stability are also confirmed by the calculated reduction energy and the density of states between HE-PBA and KMn[Fe(CN)6] (KMnHCF). This work not only presents a high-performance HE-PBA cathode in ZIBs, but also introduces a novel concept of high entropy benefiting for designing advanced materials.

Similar content being viewed by others
References
Cao, L. S.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C. Y.; Chen, L.; Vatamanu, J.; Hu, E. Y.; Hourwitz, M. J. et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat. Nanotechnol. 2021, 16, 902–910.
Liang, Y. L.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656.
Zhang, Q.; Luan, J. Y.; Tang, Y. G.; Ji, X. B.; Wang, H. Y. Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew. Chem., Int. Ed. 2020, 59, 13180–13191.
Huang, J. T.; Zhou, J.; Liang, S. Q. Guest pre-intercalation strategy to boost the electrochemical performance of aqueous zinc-ion battery Cathodes. Acta Phys. Chim. Sin. 2021, 37, 27–49.
He, Y. N.; Xu, Y. F.; Zhang, M.; Xu, J. Z.; Chen, B. B.; Zhang, Y. X.; Bao, J. C.; Zhou, X. S. Confining ultrafine SnS nanoparticles in hollow multichannel carbon nanofibers for boosting potassium storage properties. Sci. Bull. 2022, 67, 151–160.
Liao, J. Y.; Chen, C. L.; Hu, Q.; Du, Y. C.; He, Y. N.; Xu, Y. F.; Zhang, Z. Z.; Zhou, X. S. A low-strain phosphate cathode for high-rate and ultralong cycle-life potassium-ion batteries. Angew. Chem., Int. Ed. 2021, 60, 25575–25582.
Gao, W. L.; Michalicka, J.; Pumera, M. Hierarchical atomic layer deposited V2O5 on 3D printed nanocarbon electrodes for high-performance aqueous zinc-ion batteries. Small 2022, 18, 2105572.
Tian, Y. P.; Ju, M. M.; Bin, X. Q.; Luo, Y. J.; Que, W. X. Long cycle life aqueous rechargeable battery Zn/Vanadium hexacyanoferrate with H+Zn2+ coinsertion for high capacity. Chem. Eng. J. 2022, 430, 132964.
Cao, T.; Zhang, F.; Chen, M. J.; Shao, T.; Li, Z.; Xu, Q. J.; Cheng, D. H.; Liu, H. M.; Xia, Y. Y. Cubic manganese potassium hexacyanoferrate regulated by controlling of the water and defects as a high-capacity and stable cathode material for rechargeable aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 26924–26935.
Jia, X. X.; Liu, C. F.; Neale, Z. G.; Yang, J. H.; Cao, G. Z. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866.
Liao, Y. X.; Chen, H. C.; Yang, C.; Liu, R.; Peng, Z. W.; Cao, H. J.; Wang, K. K. Unveiling performance evolution mechanisms of MnO2 polymorphs for durable aqueous zinc-ion batteries. Energy Stor. Mater. 2022, 44, 508–516.
Jin, Y.; Zou, L. F.; Liu, L. L.; Engelhard, M. H.; Patel, R. L.; Nie, Z. M.; Han, K. S.; Shao, Y. Y.; Wang, C. M.; Zhu, J. et al. Joint charge storage for high-rate aqueous zinc-manganese dioxide batteries. Adv. Mater. 2019, 31, 1900567.
Zhong, C.; Liu, B.; Ding, J.; Liu, X. R.; Zhong, Y. W.; Li, Y.; Sun, C. B.; Han, X. P.; Deng, Y. D.; Zhao, N. Q. et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat. Energy 2020, 5, 440–449.
Zhang, Y. R.; Chen, A. B.; Sun, J. Promise and challenge of vanadium-based cathodes for aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 655–667.
Wan, F.; Niu, Z. Q. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew. Chem., Int. Ed. 2019, 58, 16358–16367.
Feng, Z. Y.; Sun, J. J.; Liu, Y. Y.; Jiang, H. M.; Cui, M.; Hu, T.; Meng, C. G.; Zhang, Y. F. Engineering interlayer space of vanadium oxide by pyridinesulfonic acid-assisted intercalation of polypyrrole enables enhanced aqueous zinc-ion storage. ACS Appl. Mater. Interfaces 2021, 13, 61154–61165.
Li, W. J.; Han, C.; Cheng, G.; Chou, S. L.; Liu, H. K.; Dou, S. X. Chemical properties, structural properties, and energy storage applications of prussian blue analogues. Small 2019, 15, 1900470.
Shi, Y. C.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y. M.; Li, Y. H.; Sun, Z. H.; Ali, W. et al. An overview and future perspectives of rechargeable zinc batteries. Small 2020, 16, 2000730.
Liu, H. Y.; Wang, J. G.; You, Z. Y.; Wei, C. G.; Kang, F. Y.; Wei, B. Q. Rechargeable aqueous zinc-ion batteries: Mechanism, design strategies and future perspectives. Mater. Today 2021, 42, 73–98.
Yi, H. C.; Qin, R. Z.; Ding, S. X.; Wang, Y. T.; Li, S. N.; Zhao, Q. H.; Pan, F. Structure and properties of prussian blue analogues in energy storage and conversion applications. Adv. Funct. Mater. 2021, 31, 2006970.
Du, G. Y.; Pang, H. Recent advancements in Prussian blue analogues: Preparation and application in batteries. Energy Stor. Mater. 2021, 36, 387–408.
Xu, Y. F.; Du, Y. C.; Yi, Z. Y.; Zhang, Z. Z.; Lai, C. L.; Liao, J. Y.; Zhou, X. S. Coupling Co3[Co(CN)6]2 nanocubes with reduced graphene oxide for high-rate and long-cycle-life potassium storage. J. Energy Chem. 2021, 58, 593–601.
Xu, J. Y.; Xu, Y. F.; Lai, C. L.; Xia, T. T.; Zhang, B. N.; Zhou, X. S. Challenges and perspectives of covalent organic frameworks for advanced alkali-metal ion batteries. Sci. China Chem. 2021, 64, 1267–1282.
Song, M.; Tan, H.; Chao, D. L.; Fan, H. J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564.
Li, H. F.; Ma, L. T.; Han, C. P.; Wang, Z. F.; Liu, Z. X.; Tang, Z. J.; Zhi, C. Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587.
Tang, B. Y.; Shan, L. T.; Liang, S. Q.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304.
Zeng, Y. X.; Lu, X. F.; Zhang, S. L.; Luan, D. Y.; Li, S.; Lou, X. W. Construction of Co-Mn prussian blue analog hollow spheres for efficient aqueous Zn-ion batteries. Angew. Chem., Int. Ed. 2021, 60, 22189–22194.
Ma, Y. J.; Ma, Y.; Dreyer, S. L.; Wang, Q. S.; Wang, K.; Goonetilleke, D.; Omar, A.; Mikhailova, D.; Hahn, H.; Breitung, B. et al. High-entropy metal-organic frameworks for highly reversible sodium storage. Adv Mater 2021, 33, 2101342.
George, E. P.; Raabe, D.; Ritchie, R. O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534.
Xie, B. X.; Zuo, P. J.; Wang, L. G.; Wang, J. J.; Huo, H.; He, M. X.; Shu, J.; Li, H. F.; Lou, S. F.; Yin, G. P. Achieving long-life Prussian blue analogue cathode for Na-ion batteries via triple-cation lattice substitution and coordinated water capture. Nano Energy 2019, 61, 201–210.
Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309.
Wang, Q. S.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S. S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T. et al. Multi-anionic and -cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ. Sci. 2019, 12, 2433–2442.
Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; De Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S. S.; Hahn, H. et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400.
Ma, Y. J.; Ma, Y.; Wang, Q. S.; Schweidler, S.; Botros, M.; Fu, T. T.; Hahn, H.; Brezesinski, T.; Breitung, B. High-entropy energy materials: Challenges and new opportunities. Energy Environ. Sci. 2021, 14, 2883–2905.
Zhao, C. L.; Ding, F. X.; Lu, Y. X.; Chen, L. Q.; Hu, Y. S. High-entropy layered oxide cathodes for sodium-ion batteries. Angew. Chem., Int. Ed. 2020, 59, 264–269.
You, Y.; Wu, X. L.; Yin, Y. X.; Guo, Y. G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647.
Ji, Z.; Han, B.; Liang, H. T.; Zhou, C. G.; Gao, Q.; Xia, K. S.; Wu, J. P. On the Mechanism of the improved operation voltage of rhombohedral nickel hexacyanoferrate as cathodes for sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 33619–33625.
Li, J. H.; He, L. Z.; Jiang, J. B.; Xu, Z. F.; Liu, M. Q.; Liu, X.; Tong, H. X.; Liu, Z.; Qian, D. Facile syntheses of bimetallic Prussian blue analogues (KxM[Fe(CN)6]•nH2O, M=Ni, Co, and Mn) for electrochemical determination of toxic 2-nitrophenol. Electrochim. Acta 2020, 353, 136579.
Chong, S. K.; Yang, J.; Sun, L.; Guo, S. W.; Liu, Y. N.; Liu, H. K. Potassium nickel iron hexacyanoferrate as ultra-long-life cathode material for potassium-ion batteries with high energy density. ACS Nano 2020, 14, 9807–9818.
Huang, Y. X.; Xie, M.; Wang, Z. H.; Jiang, Y.; Yao, Y.; Li, S. J.; Li, Z. H.; Li, L.; Wu, F.; Chen, R. J. A chemical precipitation method preparing hollow-core-shell heterostructures based on the prussian blue analogs as cathode for sodium-ion batteries. Small 2018, 14, 1801246.
Zhao, C. X.; Liu, B.; Li, X. N.; Zhu, K. X.; Hu, R. S.; Ao, Z. M.; Wang, J. H. A Co-Fe Prussian blue analogue for efficient Fenton-like catalysis: The effect of high-spin cobalt. Chem. Commun. 2019, 55, 7151–7154.
Bie, X. F.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. Synthesis and electrochemical properties of Na-rich Prussian blue analogues containing Mn, Fe, Co, and Fe for Na-ion batteries. J. Power Sources 2018, 378, 322–330.
Li, Q.; Ma, K. X.; Yang, G. Z.; Wang, C. X. High-voltage non-aqueous Zn/K1.6Mn1.2Fe(CN)6 batteries with zero capacity loss in extremely long working duration. Energy Stor. Mater. 2020, 29, 246–253.
Xia, M. T.; Zhang, X. K.; Liu, T. T.; Yu, H. X.; Chen, S.; Peng, N.; Zheng, R. T.; Zhang, J. D.; Shu, J. Commercially available Prussian blue get energetic in aqueous K-ion batteries. Chem. Eng. J. 2020, 394, 124923.
Ma, L. T.; Chen, S. M.; Long, C. B.; Li, X. L.; Zhao, Y. W.; Liu, Z. X.; Huang, Z. D.; Dong, B. B.; Zapien, J. A.; Zhi, C. Y. Achieving high-voltage and high-capacity aqueous rechargeable zinc ion battery by incorporating two-species redox reaction. Adv. Energy Mater. 2019, 9, 1902446.
Tao, Y. Y.; Li, Z.; Tang, L. B.; Pu, X. M.; Cao, T.; Cheng, D. H.; Xu, Q. J.; Liu, H. M.; Wang, Y. G.; Xia, Y. Y. Nickel and cobalt Co-substituted spinel ZnMn2O4@N-rGO for increased capacity and stability as a cathode material for rechargeable aqueous zinc-ion battery. Electrochim. Acta 2020, 331, 135296.
Tang, Y.; Li, W.; Feng, P. Y.; Zhou, M.; Wang, K. L.; Wang, Y. S.; Zaghib, K.; Jiang, K. High-performance manganese hexacyanoferrate with cubic structure as superior cathode material for sodium-ion batteries. Adv. Funct. Mater. 2020, 30, 1908754.
Gao, Y. N.; Yang, H. Y.; Wang, X. R.; Bai, Y.; Zhu, N.; Guo, S. N.; Suo, L. M.; Li, H.; Xu, H. J.; Wu, C. The compensation effect mechanism of Fe-Ni mixed prussian blue analogues in aqueous rechargeable aluminum-ion batteries. ChemSusChem 2020, 13, 732–740.
Xu, Y.; Wan, J.; Huang, L.; Xu, J.; Ou, M. Y.; Liu, Y.; Sun, X. P.; Li, S.; Fang, C.; Li, Q. et al. Dual redox-active copper hexacyanoferrate nanosheets as cathode materials for advanced sodium-ion batteries. Energy Stor. Mater. 2020, 33, 432–441.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Henkelman, G.; Uberuaga, B. P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904.
Acknowledgements
The authors sincerely acknowledge the financial support received from the National Natural Science Foundation of China (Nos. 21908204, 52074244, 2022TQ0285 and 52206282) and the Center of Advanced Analysis & Computational Science, Zhengzhou University for their characterization.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xing, J., Zhang, Y., Jin, Y. et al. Active cation-integration high-entropy Prussian blue analogues cathodes for efficient Zn storage. Nano Res. 16, 2486–2494 (2023). https://doi.org/10.1007/s12274-022-5020-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5020-0