Abstract
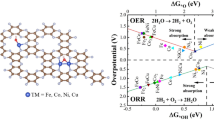
Dual-metal catalysts with synergistic effect exhibit enormous potential for sustainable electrocatalytic applications and mechanism research. Compared with mono-metal-site catalysts, dual-metal-site catalysts exhibit higher efficiency for the oxygen evolution reaction (OER) due to reduced energy barrier of the process involving proton-coupled multi-electron transfer. Herein, we construct dual-metal Fe-Co sites coordinated with nitrogen in graphene (FeCo-NG), which exhibits high OER performance with onset overpotential of only 126 mV and Tafel slope of 120 mV·dec−1, showing that the rate-determining step is controlled by the single-electron transfer step. Theoretical calculations reveal that the FeN4 site exhibits lower OER overpotential than the CoN4 site due to appropriate adsorption energy of OOH* on the former, while the O* adsorbed on the adjacent Co site could stabilize the OOH* on the FeN4 site through hydrogen bond interaction.

Similar content being viewed by others
References
Guan, J. Q.; Bai, X.; Tang, T. M. Recent progress and prospect of carbon-free single-site catalysts for the hydrogen and oxygen evolution reactions. Nano Res. 2022, 15, 818–837.
Tang, T. M.; Li, S. S.; Sun, J. R.; Wang, Z. L.; Guan, J. Q. Advances and challenges in two-dimensional materials for oxygen evolution. Nano Res., in press, https://doi.org/10.1007/s12274-022-4575-0.
Zhang, Q. Q.; Guan, J. Q. Atomically dispersed catalysts for hydrogen/oxygen evolution reactions and overall water splitting. J. Power Sources 2020, 471, 228446.
Zheng, X. B.; Chen, Y. P.; Lai, W. H.; Li, P.; Ye, C. L.; Liu, N. N.; Dou, S. X.; Pan, H. G.; Sun, W. P. Enriched d-band holes enabling fast oxygen evolution kinetics on atomic-layered defect-rich lithium cobalt oxide nanosheets. Adv. Funct. Mater. 2022, 32, 2200663.
Zhang, Q. Q.; Guan, J. Q. Applications of single-atom catalysts. Nano Res. 2022, 15, 38–70.
Tang, T. M.; Wang, Z. L.; Guan, J. Q. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Catal. 2022, 43, 636–678.
Bai, X.; Wang, L. M.; Nan, B.; Tang, T. M.; Niu, X. D.; Guan, J. Q. Atomic manganese coordinated to nitrogen and sulfur for oxygen evolution. Nano Res. 2022, 15, 6019–6025.
Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514.
Han, L.; Dong, S. J.; Wang, E. K. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291.
Sultan, S.; Tiwari, J. N.; Singh, A. N.; Zhumagali, S.; Ha, M. R.; Myung, C. W.; Thangavel, P.; Kim, K. S. Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting. Adv. Energy Mater. 2019, 9, 1900624.
Bai, X.; Duan, Z. Y.; Nan, B.; Wang, L. M.; Tang, T. M.; Guan, J. Q. Unveiling the active sites of ultrathin Co-Fe layered double hydroxides for the oxygen evolution reaction. Chin. J. Catal. 2022, 43, 2240–2248.
Smith, R. D. L.; Pasquini, C.; Loos, S.; Chernev, P.; Klingan, K.; Kubella, P.; Mohammadi, M. R.; Gonzalez-Flores, D.; Dau, H. Spectroscopic identification of active sites for the oxygen evolution reaction on iron-cobalt oxides. Nat. Commun. 2017, 8, 2022.
Zhuang, L. Z.; Ge, L.; Yang, Y. S.; Li, M. R.; Jia, Y.; Yao, X. D.; Zhu, Z. H. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793.
Enman, L. J.; Stevens, M. B.; Dahan, M. H.; Nellist, M. R.; Toroker, M. C.; Boettcher, S. W. Operando X-ray absorption spectroscopy shows iron oxidation is concurrent with oxygen evolution in cobalt-iron (oxy)hydroxide electrocatalysts. Angew. Chem., Int. Ed. 2018, 57, 12840–12844.
Gong, L.; Chng, X. Y. E.; Du, Y. H.; Xi, S. B.; Yeo, B. S. Enhanced catalysis of the electrochemical oxygen evolution reaction by iron(III) Ions adsorbed on amorphous cobalt oxide. ACS Catal. 2018, 8, 807–814.
Subbaraman, R.; Tripkovic, D.; Chang, K. C.; Strmcnik, D.; Paulikas, A. P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N. M. Trends in activity for the water electrolyser reactions on 3d M(Ni, Co, Fe, Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557.
Bai, L. C.; Hsu, C. S.; Alexander, D. T. L.; Chen, H. M.; Hu, X. L. A cobalt-iron double-atom catalyst for the oxygen evolution reaction. J. Am. Chem. Soc. 2019, 141, 14190–14199.
Wu, C. C.; Zhang, X. M.; Xia, Z. X.; Shu, M.; Li, H. Q.; Xu, X. L.; Si, R.; Rykov, A. I.; Wang, J. H.; Yu, S. S. et al. Insight into the role of Ni-Fe dual sites in the oxygen evolution reaction based on atomically metal-doped polymeric carbon nitride. J. Mater. Chem. A 2019, 7, 14001–14010.
Zhao, X. M.; Liu, X.; Huang, B. Y.; Wang, P.; Pei, Y. Hydroxyl group modification improves the electrocatalytic ORR and OER activity of graphene supported single and bi-metal atomic catalysts (Ni, Co, and Fe). J. Mater. Chem. A 2019, 7, 24583–24593.
Zhang, Q. Q.; Guan, J. Q. Applications of atomically dispersed oxygen reduction catalysts in fuel cells and zinc-air batteries. Energy Environ. Mater. 2021, 4, 307–335.
Tang, T. M.; Wang, Z. L.; Guan, J. Q. Optimizing the electrocatalytic selectivity of carbon dioxide reduction reaction by regulating the electronic structure of single-atom M-N-C materials. Adv. Funct. Mater. 2022, 32, 2111504.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013.
Wang, X. W.; Qiu, S. Y.; Feng, J. M.; Tong, Y. Y.; Zhou, F. L.; Li, Q. Y.; Song, L.; Chen, S. M.; Wu K. H.; Su P. P. et al. Confined Fe-Cu clusters as sub-nanometer reactors for efficiently regulating the electrochemical nitrogen reduction reaction. Adv. Mater. 2020, 32, 2004382.
Zheng, X. B.; Li, B. B.; Wang, Q. S.; Wang, D. S.; Li, Y. D. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis. Nano Res. 2022, 15, 7806–7839.
Zheng, X. B.; Yang, J. R.; Xu, Z. F.; Wang, Q. S.; Wu, J. B.; Zhang, E. H.; Dou, S. X.; Sun, W. P.; Wang, D. S.; Li, Y. D. Ru-Co pair sites catalyst boosts the energetics for the oxygen evolution reaction. Angew. Chem., Int. Ed. 2022, 134, e202205946.
Han, J. Y.; Zhang, M. Z.; Bai, X.; Duan, Z. Y.; Tang, T. M.; Guan, J. Q. Mesoporous Mn-Fe oxyhydroxides for oxygen evolution. Inorg. Chem. Front. 2022, 9, 3559–3565.
Wan, W. C.; Zhao, Y. G.; Wei, S. Q.; Triana, C. A.; Li, J. G.; Arcifa, A.; Allen, C. S.; Cao, R.; Patzke, G. R. Mechanistic insight into the active centers of single/dual-atom Ni/Fe-based oxygen electrocatalysts. Nat. Commun. 2021, 12, 5589.
Nechiyil, D.; Vinayan, B. P.; Ramaprabhu, S. Tri-iodide reduction activity of ultra-small size PtFe nanoparticles supported nitrogen-doped graphene as counter electrode for dye-sensitized solar cell. J. Colloid Interface Sci. 2017, 488, 309–316.
Ma, L. T.; Chen, S. M.; Pei, Z. X.; Huang, Y.; Liang, G. J.; Mo, F. N.; Yang, Q.; Su, J.; Gao, Y. H.; Zapien, J. A. et al. Single-site active iron-based bifunctional oxygen catalyst for a compressible and rechargeable zinc-air battery. ACS Nano 2018, 12, 1949–1958.
Zhao, S. Y.; Zhang, L. J.; Johannessen, B.; Saunders, M.; Liu, C.; Yang, S. Z.; Jiang, S. P. Designed iron single atom catalysts for highly efficient oxygen reduction reaction in alkaline and acid media. Adv. Mater. Interfaces 2021, 8, 2001788.
Guo, X. M.; Liu, S. J.; Wan, X. H.; Zhang, J. H.; Liu, Y. J.; Zheng, X. J.; Kong, Q. H.; Jin, Z. Controllable solid-phase fabrication of an Fe2O3/Fe5C2/Fe-N-C electrocatalyst toward optimizing the oxygen reduction reaction in zinc-air batteries. Nano Lett. 2022, 22, 4879–4887.
Li, F.; Qin, T. T.; Sun, Y. P.; Jiang, R. J.; Yuan, J. F.; Liu, X. Q.; O’Mullane, A. P. Preparation of a one-dimensional hierarchical MnO@CNT@Co-N/C ternary nanostructure as a high-performance bifunctional electrocatalyst for rechargeable Zn-air batteries. J. Mater. Chem. A 2021, 9, 22533–22543.
Guan, J. Q.; Duan, Z. Y.; Zhang, F. X.; Kelly, S. D.; Si, R.; Dupuis, M.; Huang, Q. G.; Chen, J. Q.; Tang, C. H.; Li, C. Water oxidation on a mononuclear manganese heterogeneous catalyst. Nat. Catal. 2018, 1, 870–877.
Li, J. K.; Jiao, L.; Wegener, E.; Richard, L. L.; Liu, E. S.; Zitolo, A.; Sougrati, M. T.; Mukerjee, S.; Zhao, Z. P.; Huang, Y. et al. Evolution pathway from iron compounds to Fe1(II)-N4 sites through gas-phase iron during pyrolysis. J. Am. Chem. Soc. 2020, 142, 1417–1423.
Suen, N. T.; Hung, S. F.; Quan, Q.; Zhang, N.; Xu, Y. J.; Chen, H. M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365.
Shen, T.; Huang, X. X.; Xi, S. B.; Li, W.; Sun, S. N.; Hou, Y. L. The ORR electron transfer kinetics control via Co-Nx and graphitic N sites in cobalt single atom catalysts in alkaline and acidic media. J. Energy Chem. 2022, 68, 184–194.
Wu, M. J.; Wei, Q. L.; Zhang, G. X.; Qiao, J. L.; Wu, M. X.; Zhang, J. H.; Gong, Q. J.; Sun, S. H. Fe/Co double hydroxide/oxide nanoparticles on N-doped CNTs as highly efficient electrocatalyst for rechargeable liquid and quasi-solid-state zinc-air batteries. Adv. Energy Mater. 2018, 8, 1801836.
Zhang, K. K.; Mai, W. S.; Li, J.; Li, G. Q.; Tian, L. H.; Hu, W. Bimetallic Co3.2Fe0. 8N-nitrogen-carbon nanocomposites for simultaneous electrocatalytic oxygen reduction, oxygen evolution, and hydrogen evolution. ACS Appl. Nano Mater. 2019, 2, 5931–5941.
Du, Q. G.; Su, P. P.; Cao, Z. Z.; Yang, J.; Price, C. A. H.; Liu, J. Construction of N and Fe co-doped CoO/CoxN interface for excellent OER performance. Sustainable Mater. Technol. 2021, 29, e00293.
Singh, T. I.; Rajeshkhanna, G.; Pan, U. N.; Kshetri, T.; Lin, H.; Kim, N. H.; Lee, J. H. Alkaline water splitting enhancement by MOF-derived Fe-Co-oxide/Co@NC-mNS heterostructure: Boosting OER and HER through defect engineering and in situ oxidation. Small 2021, 17, 2101312.
Chen, Y.; Hu, S. Q.; Nichols, F.; Bridges, F.; Kan, S. T.; He, T.; Zhang, Y.; Chen, S. W. Carbon aerogels with atomic dispersion of binary iron-cobalt sites as effective oxygen catalysts for flexible zinc-air batteries. J. Mater. Chem. A 2020, 8, 11649–11655.
Gao, T. T.; Jin, Z. Y.; Zhang, Y. J.; Tan, G. Q.; Yuan, H. Y.; Xiao, D. Coupling cobalt-iron bimetallic nitrides and N-doped multi-walled carbon nanotubes as high-performance bifunctional catalysts for oxygen evolution and reduction reaction. Electrochim. Acta 2017, 258, 51–60.
Wang, W. G.; Babu, D. D.; Huang, Y. Y.; Lv, J. Q.; Wang, Y. B.; Wu, M. X. Atomic dispersion of Fe/Co/N on graphene by ball-milling for efficient oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 10351–10358.
Tan, W.; Xie, S. Q.; Yang, J. W.; Lv, J. G.; Yin, J. F.; Zhang, C.; Wang, J. Y.; Shen, X. Y.; Zhao, M.; Zhang, M. et al. Effect of carbonization temperature on electrocatalytic water splitting of Fe-Co anchored on N-doped porous carbon. J. Solid State Chem. 2021, 302, 122435.
Yin, P. Q.; Yao, T.; Wu, Y. E.; Zheng, L. R.; Lin, Y.; Liu, W.; Ju, H. X.; Zhu, J. F.; Hong, X.; Deng, Z. X. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22075099 and 31971322), the Education Department of Jilin Province (Nos. JJKH20220967KJ and JJKH20220968CY), the Natural Science Foundation of Jilin Province (No. 20220101051JC), the Natural Science Foundation of Shaanxi Province (No. D5110220052), the Fundamental Research Funds for the Central Universities (No. D5000210743), and Beijing Municipal Health Commission (No. 2021-1G-1191).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tang, T., Duan, Z., Baimanov, D. et al. Synergy between isolated Fe and Co sites accelerates oxygen evolution. Nano Res. 16, 2218–2223 (2023). https://doi.org/10.1007/s12274-022-5001-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5001-3