Abstract
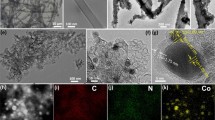
The preparation of supported high-density metal nanoparticles (NPs) is of great importance to boost the performance in heterogeneous catalysis. Thermal transformation of metal-organic frameworks (MOFs) has been demonstrated as a promising route for the synthesis of supported metal NPs with high metal loadings, but it is challenge to achieve uniform metal dispersion. Here we report a strategy of “spatial isolation and dopant anchoring” to resist metal aggregation in the pyrolysis of MOFs through converting a bulk MOF into dual-heteroatom-containing flower-like MOF sheets (B/N-MOF-S). This approach can spatially isolate metal ions and increase the number of anchoring sites, thus efficiently building physical and/or chemical barriers to cooperatively prevent metal NPs from aggregation in the high-temperature transformation process. After thermolysis at 1,000 °C, the B/N-MOFS affords B,N co-doped carbon-supported Co NPs (Co/BNC) with uniform dispersion and a high Co loading of 37.3 wt.%, while untreated bulk MOFs yield much larger sizes and uneven distribution of Co NPs. The as-obtained Co/BNC exhibits excellent electrocatalytic activities in both hydrogen evolution and hydrazine oxidation reactions, and only a voltage of 0.617 V at a high current density of 100 mA·cm−2 is required when applied to a two-electrode overall hydrazine splitting electrolyzer.

Similar content being viewed by others
References
White, R. J.; Luque, R.; Budarin, V. L.; Clark, J. H.; Macquarrie, D. J. Supported metal nanoparticles on porous materials. Methods and applications.. Chem. Soc. Rev. 2009, 38, 481–494.
Gao, C. B.; Lyu, F. L.; Yin, Y. D. Encapsulated metal nanoparticles for catalysis. Chem. Rev. 2021, 121, 834–881.
Wang, L. X.; Wang, L.; Meng, X. J.; Xiao, F. S. New strategies for the preparation of sinter-resistant metal-nanoparticle-based catalysts. Adv. Mater. 2019, 31, 1901905.
Li, X. H.; Antonietti, M. Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: Functional Mott-Schottky heterojunctions for catalysis. Chem. Soc. Rev. 2013, 42, 6593–6604.
Yin, H. J.; Tang, H. J.; Wang, D.; Gao, Y.; Tang, Z. Y. Facile synthesis of surfactant-free Au cluster/graphene hybrids for highperformance oxygen reduction reaction. ACS Nano 2012, 6, 8288–8297.
Wu, Y. C.; Wei, W.; Yu, R. H.; Xia, L. X.; Hong, X. F.; Zhu, J. X.; Li, J. T.; Lv, L.; Chen, W.; Zhao, Y. et al. Anchoring sub-nanometer Pt clusters on crumpled paper-like MXene enables high hydrogen evolution mass activity. Adv. Funct. Mater. 2022, 32, 2110910.
Chen, S. H.; Li, W. H.; Jiang, W. J.; Yang, J. R.; Zhu, J. X.; Wang, L. Q.; Ou, H. H.; Zhuang, Z. C.; Chen, M. Z.; Sun, X. H. et al. MOF encapsulating N-heterocyclic carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis. Angew. Chem., Int. Ed. 2022, 61, e202114450.
Zhu, J. X.; Xia, L. X.; Yang, W. X.; Yu, R. H.; Zhang, W.; Luo, W.; Dai, Y. H.; Wei, W.; Zhou, L.; Zhao, Y.; Mai, L. Q. Activating inert sites in cobalt silicate hydroxides for oxygen evolution through atomically doping. Energy Environ. Mater. 2022, 5, 655–661.
Furukawa, H.; Cordova, K. E.; O'Keeffe, M.; Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444.
Kitagawa, S.; Kitaura, R.; Noro, S. I. Functional porous coordination polymers. Angew. Chem., Int. Ed. 2004, 43, 2334–2375.
Ye, Y. X.; Gong, L. S.; Xiang, S. C.; Zhang, Z. J.; Chen, B. L. Metalorganic frameworks as a versatile platform for proton conductors. Adv. Mater. 2020, 32, 1907090.
Wang, H. F.; Chen, L. Y.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448.
Dang, S.; Zhu, Q. L.; Xu, Q. Nanomaterials derived from metalorganic frameworks. Nat. Rev. Mater. 2018, 3, 17075.
Chen, L. Y.; Wang, H. F.; Li, C. X.; Xu, Q. Bimetallic metal-organic frameworks and their derivatives. Chem. Sci. 2020, 11, 5369–5403.
Cai, G. R.; Yan, P.; Zhang, L. L.; Zhou, H. C.; Jiang, H. L. Metalorganic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278–12326.
Shen, K.; Chen, X. D.; Chen, J. Y.; Li, Y. W. Development of MOFderived carbon-based nanomaterials for efficient catalysis. ACS Catal. 2016, 6, 5887–5903.
Jagadeesh, R. V.; Murugesan, K.; Alshammari, A. S.; Neumann, H.; Pohl, M. M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332.
Wang, C. H.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J. S.; Yamauchi, Y. New strategies for novel MOFderived carbon materials based on nanoarchitectures. Chem 2020, 6, 19–40.
Han, X. P.; Ling, X. F.; Wang, Y.; Ma, T. Y.; Zhong, C.; Hu, W. B.; Deng, Y. D. Generation of nanoparticle, atomic-cluster, and singleatom cobalt catalysts from zeolitic imidazole frameworks by spatial isolation and their use in zinc-air batteries. Angew. Chem., Int. Ed. 2019, 58, 5359–5364.
Guan, B. Y.; Yu, L.; Lou, X. W. A dual-metal-organic-framework derived electrocatalyst for oxygen reduction. Energy Environ. Sci. 2016, 9, 3092–3096.
Yin, P. Q.; Yao, T.; Wu, Y. E.; Zheng, L. R.; Lin, Y.; Liu, W.; Ju, H. X.; Zhu, J. F.; Hong, X.; Deng, Z. X. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805.
Chen, Y. Z.; Wang, C. M.; Wu, Z. Y.; Xiong, Y. J.; Xu, Q.; Yu, S. H.; Jiang, H. L. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016.
Wang, R.; Dong, X. Y.; Du, J.; Zhao, J. Y.; Zang, S. Q. MOF-derived bifunctional Cu3P nanoparticles coated by a N,P-codoped carbon shell for hydrogen evolution and oxygen reduction. Adv. Mater. 2018, 30, 1703711.
Tabassum, H.; Guo, W. H.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q. F.; Zou, R. Q. Metal-organic frameworks derived cobalt phosphide architecture encapsulated into B/N co-doped graphene nanotubes for all pH value electrochemical hydrogen evolution. Adv. Energy Mater. 2017, 7, 1601671.
Yang, J. L.; Ju, Z. C.; Jiang, Y.; Xing, Z.; Xi, B. J.; Feng, J. K.; Xiong, S. L. Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassium-ion storage. Adv. Mater. 2018, 30, 1700104.
Yang, L.; Zeng, X. F.; Wang, W. C.; Cao, D. P. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells. Adv. Funct. Mater. 2018, 28, 1704537.
Yao, W.; Chen, J. M.; Wang, Y. J.; Fang, R. Q.; Qin, Z.; Yang, X. F.; Chen, L. Y.; Li, Y. W. Nitrogen-doped carbon composites with ordered macropores and hollow walls. Angew. Chem., Int. Ed. 2021, 60, 23729–23734.
Zhang, M. D.; Dai, Q. B.; Zheng, H. G.; Chen, M. D.; Dai, L. M. Novel MOF-derived Co@N-C bifunctional catalysts for highly efficient Zn-air batteries and water splitting. Adv. Mater. 2018, 30, 1705431.
Ji, D. X.; Fan, L.; Tao, L.; Sun, Y. J.; Li, M. G.; Yang, G. R.; Tran, T. Q.; Ramakrishna, S.; Guo, S. J. The kirkendall effect for engineering oxygen vacancy of hollow Co3O4 nanoparticles toward high-performance portable zinc-air batteries. Angew. Chem., Int. Ed. 2019, 58, 13840–13844.
Cao, L.; Dai, P. C.; Tang, J.; Li, D.; Chen, R. H.; Liu, D. D.; Gu, X.; Li, L. J.; Bando, Y.; Ok, Y. S. et al. Spherical superstructure of boron nitride nanosheets derived from boron-containing metalorganic frameworks. J. Am. Chem. Soc. 2020, 142, 8755–8762.
Wang, X. F.; Zhang, F. F.; Gao, L.; Yang, Z. H.; Pan, S. L. Nontoxic KBBF family member Zn2BO3(OH): Balance between beneficial layered structure and layer tendency. Adv. Sci. 2019, 6, 1901679.
Kou, Z. K.; Guo, B. B.; He, D. P.; Zhang, J.; Mu, S. C. Transforming two-dimensional boron carbide into boron and chlorine dual-doped carbon nanotubes by chlorination for efficient oxygen reduction. ACS Energy Lett. 2018, 3, 184–190.
Chen, S. C.; Chen, Z. H.; Siahrostami, S.; Higgins, D.; Nordlund, D.; Sokaras, D.; Kim, T. R.; Liu, Y. Z.; Yan, X. Z.; Nilsson, E. et al. Designing boron nitride islands in carbon materials for efficient electrochemical synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2018, 140, 7851–7859.
Xia, Q. Y.; Yang, H.; Wang, M.; Yang, M.; Guo, Q. B.; Wan, L. M.; Xia, H.; Yu, Y. High energy and high power lithium-ion capacitors based on boron and nitrogen dual-doped 3D carbon nanofibers as both cathode and anode. Adv. Energy Mater. 2017, 7, 1701336.
Zhang, Y. Q.; Zhou, B.; Wei, Z. X.; Zhou, W.; Wang, D. D.; Tian, J.; Wang, T. H.; Zhao, S. L.; Liu, J. L.; Tao, L. et al. Coupling glucose-assisted Cu(I)/Cu(II) redox with electrochemical hydrogen production. Adv. Mater. 2021, 33, 2104791.
Wang, H. F.; Tang, C.; Zhang, Q. A review of precious-metal-free bifunctional oxygen electrocatalysts: Rational design and applications in Zn-air batteries. Adv. Funct. Mater. 2018, 28, 1803329.
Tang, C.; Zhang, R.; Lu, W. B.; Wang, Z.; Liu, D. N.; Hao, S.; Du, G.; Asiri, A. M.; Sun, X. P. Energy-saving electrolytic hydrogen generation: Ni2P nanoarray as a high-performance non-noble-metal electrocatalyst. Angew. Chem., Int. Ed. 2017, 56, 842–846.
Sun, F.; Qin, J. S.; Wang, Z. Y.; Yu, M. Z.; Wu, X. H.; Sun, X. M.; Qiu, J. S. Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 2021, 12, 4182.
Liu, Y.; Zhang, J. H.; Li, Y. P.; Qian, Q. Z.; Li, Z. Y.; Zhu, Y.; Zhang, G. Q. Manipulating dehydrogenation kinetics through dualdoping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production. Nat. Commun. 2020, 11, 1853.
Zhu, Y.; Zhang, J. H.; Qian, Q. Z.; Li, Y. P.; Li, Z. Y.; Liu, Y.; Xiao, C.; Zhang, G. Q.; Xie, Y. Dual nanoislands on Ni/C hybrid nanosheet activate superior hydrazine oxidation-assisted high-efficiency H2 production. Angew. Chem., Int. Ed. 2022, 61, e202113082.
Zhang, J. Y.; Wang, H. M.; Tian, Y. F.; Yan, Y.; Xue, Q.; He, T.; Liu, H. F.; Wang, C. D.; Chen, Y.; Xia, B. Y. Anodic hydrazine oxidation assists energy-efficient hydrogen evolution over a bifunctional cobalt perselenide nanosheet electrode. Angew. Chem., Int. Ed. 2018, 57, 7649–7653.
Jin, H. Y.; Wang, X. S.; Tang, C.; Vasileff, A.; Li, L. Q.; Slattery, A.; Qiao, S. Z. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 2021, 33, 2007508.
Ojha, K.; Farber, E. M.; Burshtein, T. Y.; Eisenberg, D. A multidoped electrocatalyst for efficient hydrazine oxidation. Angew. Chem., Int. Ed. 2018, 57, 17168–17172.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21825802, 22138003, 22108083, and 52172142), the Foundation of Advanced Catalytic Engineering Research Center of the Ministry of Education (No. 2020AC006), the Science and Technology Program of Qingyuan City (No. 2021YFJH01002), the Natural Science Foundation of Guangdong Province (No. 2017A030312005), the Guangdong University Students Special Fund for Science and Technology Innovation Cultivation (No. pdjh2022a0031), the National Training Program of Innovation and Entrepreneurship for Undergraduates (No. 202210561050), and the Science and Technology Program of Guangzhou (No. 202201010118).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4777_MOESM1_ESM.pdf
Resisting metal aggregation in pyrolysis of MOFs towards high-density metal nanocatalysts for efficient hydrazine assisted hydrogen production
Rights and permissions
About this article
Cite this article
Ding, J., Guo, D., Hu, A. et al. Resisting metal aggregation in pyrolysis of MOFs towards high-density metal nanocatalysts for efficient hydrazine assisted hydrogen production. Nano Res. 16, 6067–6075 (2023). https://doi.org/10.1007/s12274-022-4777-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4777-5