Abstract
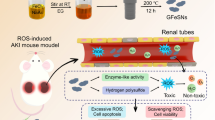
Ferroptosis plays a critical pathophysiological role in several types of acute kidney injury (AKI). The development of nanomaterials targeting iron metabolism and ferroptosis is a promising approach for AKI treatment. Herein, we synthesized gallic acid-gallium polyvinyl pyrrolidone nanoparticles (GGP NPs) as a potential iron-scavenging agent because of their nearly ionic radius and chemical similarity with iron. The results indicated that GGP NPs accumulated in tubular epithelial cells and showed good biocompatibility. GGP NPs significantly inhibited cisplatin (CP)-induced ferroptosis in HK-2 cells by reducing the accumulation of intracellular free iron and mitochondrial dysfunction, and suppressing the perturbations of ferroptosis processes, including lipid peroxidation, nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione (GSH) levels, glutathione peroxidase 4 (GPX4) activity, and ferritinophagy. An in vivo study demonstrated that treatment with GGP NPs significantly ameliorated the renal tubular injury and mitochondrial damage induced by CP treatment or ischemia-reperfusion injury. Our study suggests that GGP NPs may be an effective and promising candidate for AKI treatment and enable potential clinical translation.

Similar content being viewed by others
References
Ronco, C.; Bellomo, R.; Kellum, J. A. Acute kidney injury. Lancet 2019, 394, 1949–1964.
He, L. Y.; Wei, Q. Q.; Liu, J.; Yi, M. X.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y. M.; Liu, F. Y.; Venkatachalam, M. A. et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083.
Scholz, H.; Boivin, F. J.; Schmidt-Ott, K. M.; Bachmann, S.; Eckardt, K. U.; Scholl, U. I.; Persson, P. B. Kidney physiology and susceptibility to acute kidney injury: Implications for renoprotection. Nat. Rev. Nephrol. 2021, 17, 335–349.
Martin-Sanchez, D.; Ruiz-Andres, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Niño, M. D.; Ortega, M. R.; Egido, J.; Linkermann, A.; Ortiz, A. et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 2017, 28, 218–229.
Deng, F.; Sharma, I.; Dai, Y. B.; Yang, M.; Kanwar, Y. S. Myoinositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Invest. 2019, 129, 5033–5049.
Linkermann, A. Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int. 2016, 89, 46–57.
Guo, L. X.; Zhang, T. P.; Wang, F.; Chen, X.; Xu, H. M.; Zhou, C.; Chen, M.; Yu, F. J.; Wang, S.; Yang, D. G. et al. Targeted inhibition of Reverb-a/β limits ferroptosis to ameliorate folic acid-induced acute kidney injury. Br. J. Pharmacol. 2021, 178, 328–345.
Hu, Z. X.; Zhang, H.; Yang, S. K.; Wu, X. Q.; He, D.; Cao, K.; Zhang, W. Emerging role of ferroptosis in acute kidney injury. Oxid. Med. Cell. Longev. 2019, 2019, 8010614.
Dixon, S. J.; Lemberg, K. M.; Lamprecht, M. R.; Skouta, R.; Zaitsev, E. M.; Gleason, C. E.; Patel, D. N.; Bauer, A. J.; Cantley, A. M.; Yang, W. S. et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072.
Doll, S.; Conrad, M. Iron and ferroptosis: A still ill-defined liaison. IUBMB Life 2017, 69, 423–434.
Scindia, Y.; Dey, P.; Thirunagari, A.; Huang, L. P.; Rosin, D. L.; Floris, M.; Okusa, M. D.; Swaminathan, S. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J. Am. Soc. Nephrol. 2015, 26, 2800–2814.
Borawski, B.; Malyszko, J. Iron, ferroptosis, and new insights for prevention in acute kidney injury. Adv. Med. Sci. 2020, 65, 361–370.
Sharma, S.; Leaf, D. E. Iron chelation as a potential therapeutic strategy for AKI prevention. J. Am. Soc. Nephrol. 2019, 30, 2060–2071.
Chitambar, C. R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta 2016, 1863, 2044–2053.
Newman, R. A.; Brody, A. R.; Krakoff, I. H. Gallium nitrate (NSC-15200) induced toxicity in the rat: A pharmacologic, histopathologic and microanalytical investigation. Cancer 1979, 44, 1728–1740.
Goss, C. H.; Kaneko, Y.; Khuu, L.; Anderson, G. D.; Ravishankar, S.; Aitken, M. L.; Lechtzin, N.; Zhou, G. L.; Czyz, D. M.; McLean, K. et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520.
Chitambar, C. R. Gallium-containing anticancer compounds. Future Med. Chem. 2012, 4, 1257–1272.
Yin, H. Y.; Gao, J. J.; Chen, X. M.; Ma, B.; Yang, Z. S.; Tang, J.; Wang, B. W.; Chen, T. F.; Wang, C.; Gao, S. et al. A gallium(III) complex that engages protein disulfide isomerase A3 (PDIA3) as an anticancer target. Angew. Chem., Int. Ed. 2020, 59, 20147–20153.
Yang, C. Y.; Yu, Y. R.; Wang, X. C.; Wang, Q.; Shang, L. R. Cellular fluidic-based vascular networks for tissue engineering. Engineered Regeneration 2021, 2, 171–174.
Yang, K. K.; Yang, Z. Q.; Yu, G. C.; Nie, Z. H.; Wang, R. B.; Chen, X. Y. Polyprodrug nanomedicines: An emerging paradigm for cancer therapy. Adv. Mater. 2022, 34, 2107434.
Chen, G. P.; Zhang, H.; Wang, H.; Wang, F. Y. Immune tolerance induced by immune-homeostatic particles. Engineered Regeneration 2021, 2, 133–136.
Ma, Y. H.; Cai, F. H.; Li, Y. Y.; Chen, J. H.; Han, F.; Lin, W. Q. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact. Mater. 2020, 5, 732–743.
Wang, L. F.; Zhang, Y. J.; Li, Y. Y.; Chen, J. H.; Lin, W. Q. Recent advances in engineered nanomaterials for acute kidney injury theranostics. Nano Res. 2021, 14, 920–933.
Wang, J.; Poon, C.; Chin, D.; Milkowski, S.; Lu, V.; Hallows, K. R.; Chung, E. J. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018, 11, 5584–5595.
Bao, Y. W.; Bai, M. Q.; Zhu, H. H.; Yuan, Y.; Wang, Y.; Zhang, Y. J.; Wang, J. N.; Xie, X. S.; Yao, X.; Mao, J. H. et al. DNA demethylase Tet2 suppresses cisplatin-induced acute kidney injury. Cell Death Discov. 2021, 7, 167.
Wang, F. Q.; Jiang, X. F.; Xiang, H. J.; Wang, N.; Zhang, Y. J.; Yao, X.; Wang, P.; Pan, H.; Yu, L. F.; Cheng, Y. F. et al. An inherently kidney-targeting near-infrared fluorophore based probe for early detection of acute kidney injury. Biosens. Bioelectron. 2021, 172, 112756.
Bao, Y. W.; Yuan, Y.; Chen, J. H.; Lin, W. Q. Kidney disease models: Tools to identify mechanisms and potential therapeutic targets. Zool. Res. 2018, 39, 72–86.
Wang, J. N.; Nie, W. Y.; Xie, X. S.; Bai, M. Q.; Ma, Y. H.; Jin, L. N.; Xiao, L.; Shi, P.; Yang, Y.; Jose, P. A. et al. MicroRNA-874-3p/ADAM (A Disintegrin and Metalloprotease) 19 mediates macrophage activation and renal fibrosis after acute kidney injury. Hypertension 2021, 77, 1613–1626.
Prus, E.; Fibach, E. Flow cytometry measurement of the labile iron pool in human hematopoietic cells. Cytometry A 2008, 73, 22–27.
Yang, J. C.; Ding, L.; Yu, L. D.; Wang, Y. M.; Ge, M.; Jiang, Q. Z.; Chen, Y. Nanomedicine enables autophagy-enhanced cancer-cell ferroptosis. Sci. Bull. 2021, 66, 464–477.
Hao, Y. N.; Gao, Y. R.; Li, Y.; Fei, T.; Shu, Y.; Wan, J. H. Ultrasmall copper—gallic acid nanodots for chemodynamic therapy. Adv. Mater. Interfaces 2021, 8, 2101173.
Ahmadvand, H.; Yalameha, B.; Adibhesami, G.; Nasri, M.; Naderi, N.; Babaeenezhad, E.; Nouryazdan, N. The protective role of gallic acid pretreatment on renal ischemia-reperfusion injury in rats. Rep. Biochem. Mol. Biol. 2019, 8, 42–48.
Eslamifar, Z.; Moridnia, A.; Sabbagh, S.; Ghaffaripour, R.; Jafaripour, L.; Behzadifard, M. Ameliorative effects of Gallic acid on cisplatin-induced nephrotoxicity in rat variations of biochemistry, histopathology, and gene expression. BioMed Res. Int. 2021, 2021, 2195238.
Yang, W. S.; SriRamaratnam, R.; Welsch, M. E.; Shimada, K.; Skouta, R.; Viswanathan, V. S.; Cheah, J. H.; Clemons, P. A.; Shamji, A. F.; Clish, C. B. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331.
Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185.
Friedmann Angeli, J. P.; Schneider, M.; Proneth, B.; Tyurina, Y. Y.; Tyurin, V. A.; Hammond, V. J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191.
Bellelli, R.; Federico, G.; Matte’, A.; Colecchia, D.; Iolascon, A.; Chiariello, M.; Santoro, M.; De Franceschi, L.; Carlomagno, F. NCOA4 deficiency impairs systemic iron homeostasis. Cell Rep. 2016, 14, 411–421.
Kelson, A. B.; Carnevali, M.; Truong-Le, V. Gallium-based antiinfectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716.
Zhao, Y.; Pu, M. J.; Wang, Y. N.; Yu, L. M.; Song, X. Y.; He, Z. Y. Application of nanotechnology in acute kidney injury: From diagnosis to therapeutic implications. J. Control. Release 2021, 336, 233–251.
Zarjou, A.; Bolisetty, S.; Joseph, R.; Traylor, A.; Apostolov, E. O.; Arosio, P.; Balla, J.; Verlander, J.; Darshan, D.; Kuhn, L. C. et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J. Clin. Invest. 2013, 123, 4423–4434.
Dowdle, W. E.; Nyfeler, B.; Nagel, J.; Elling, R. A.; Liu, S. M.; Triantafellow, E.; Menon, S.; Wang, Z. C.; Honda, A.; Pardee, G. et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014, 16, 1069–1079.
Kuang, S.; Liao, X. X.; Zhang, X. R.; Rees, T. W.; Guan, R. L.; Xiong, K.; Chen, Y.; Ji, L. N.; Chao, H. FerriIridium: A lysosometargeting iron(III)-activated iridium(III) prodrug for chemotherapy in gastric cancer cells. Angew. Chem., Int. Ed. 2020, 59, 3315–3321.
Pan, J.; Harriss, B. I.; Chan, C. F.; Jiang, L. J.; Tsoi, T. H.; Long, N. J.; Wong, W. T.; Wong, W. K.; Wong, K. L. Gallium and functionalized-porphyrins combine to form potential lysosomespecific multimodal bioprobes. Inorg. Chem. 2016, 55, 6839–6841.
Gaschler, M. M.; Stockwell, B. R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425.
Conrad, M.; Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147.
Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X. X.; Freitas, F. P.; Seibt, T. et al. selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018, 172, 409–422.E21.
Maiorino, M.; Conrad, M.; Ursini, F. GPx4, lipid peroxidation, and cell death: Discoveries, rediscoveries, and open issues. Antioxid. Redox Signal. 2018, 29, 61–74.
Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (No. 2018YFC2000400), Zhejiang Provincial Natural Science Foundation of China (No. LZ22H050001), the National Natural Science Foundation of China (Nos. 81970573, 81670651, and 82000637), Zhejiang provincial program for the Cultivation of High-level Innovative Health talents, and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2020KY538).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4257_MOESM1_ESM.pdf
Targeting iron metabolism using gallium nanoparticles to suppress ferroptosis and effectively mitigate acute kidney injury
Rights and permissions
About this article
Cite this article
Xie, X., Zhang, Y., Su, X. et al. Targeting iron metabolism using gallium nanoparticles to suppress ferroptosis and effectively mitigate acute kidney injury. Nano Res. 15, 6315–6327 (2022). https://doi.org/10.1007/s12274-022-4257-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4257-y