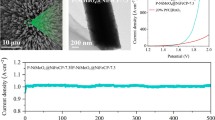
Abstract
Hierarchical-structure materials hold great promise for numerous applied domains such as fuel cell, sensor, and optic. However, the developments are significantly impeded by the lack of efficient strategy permitting precise and efficient decoration of specific confined space. Here, an in-situ precise hybridization strategy is proposed to efficiently manipulate the nanostructure of membrane nanochannels. Typically, Nafion ionic nanochannels are impregnated with precursors via heat swelling, followed by microwave-assisted condensation to form polymer quantum dot network. The formation of polymer quantum dot network significantly improves the stability and functionality of ionic nanophase (i.e., ionic nanochannel). This helps hybrid membrane achieving enhanced proton conduction and methanol barrier properties, resulting in over ten times increase in proton/methanol selectivity. These then impart prominent device performances for both hydrogen and methanol fuel cells with the elevation of ∼ 100%. Importantly, such function manipulation of ionic nanochannels is achieved with fully maintaining function of backbone nanophase. Besides, the regulation of physical topology and chemical environment of ionic nanochannel also brings optimization of gas and ion separation properties. This facile and versatile strategy may open up a new avenue for decorating confined space of many hierarchical-structure materials.

Similar content being viewed by others
References
Feng, L.; Wang, K. Y.; Day, G. S.; Zhou, H. C. The chemistry of multi-component and hierarchical framework compounds. Chem. Soc. Rev. 2019, 48, 4823–4853.
Mao, H. Y.; Tang, J.; Chen, J.; Wan, J. Y.; Hou, K. P.; Peng, Y. C.; Halat, D. M.; Xiao, L. G.; Zhang, R. F.; Lv, X. D. et al. Designing hierarchical nanoporous membranes for highly efficient gas adsorption and storage. Sci. Adv. 2020, 6, eabb0694.
Zhao, M. Q.; Zhang, Q.; Huang, J. Q.; Wei, F. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides-properties, synthesis, and applications. Adv. Funct. Mater. 2012, 22, 675–694.
Vallé, K.; Belleville, P.; Pereira, F.; Sanchez, C. Hierarchically structured transparent hybrid membranes by in situ growth of mesostructured organosilica in host polymer. Nat. Mater. 2006, 5, 107–111.
Kusoglu, A.; Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104.
Park, C. H.; Lee, S. Y.; Hwang, D. S.; Shin, D. W.; Cho, D. H.; Lee, K. H.; Kim, T. W.; Kim, T. W.; Lee, M.; Kim, D. S. et al. Nanocrack-regulated self-humidifying membranes. Nature 2016, 532, 480–483.
Qian, X. T.; Chen, L.; Yin, L. C.; Liu, Z. B.; Pei, S. F.; Li, F.; Hou, G. J.; Chen, S. G.; Song, L.; Thebo, K. H. et al. CdPS3 nanosheets-based membrane with high proton conductivity enabled by Cd vacancies. Science 2020, 370, 596–600.
Hu, S.; Lozada-Hidalgo, M.; Wang, F. C.; Mishchenko, A.; Schedin, F.; Nair, R. R.; Hill, E. W.; Boukhvalov, D. W.; Katsnelson, M. I.; Dryfe, R. A. W. et al. Proton transport through one-atom-thick crystals. Nature 2014, 516, 227–230.
Cao, L.; Wu, H.; Cao, Y.; Fan, C. Y.; Zhao, R.; He, X. Y.; Yang, P. F.; Shi, B. B.; You, X. D.; Jiang, Z. Y. Weakly humidity-dependent proton-conducting COF membranes. Adv. Mater. 2020, 32, 2005565.
Paneri, A.; Heo, Y.; Ehlert, G.; Cottrill, A.; Sodano, H.; Pintauro, P.; Moghaddam, S. Proton selective ionic graphene-based membrane for high concentration direct methanol fuel cells. J. Membr. Sci. 2014, 467, 217–225.
Wu, W. J.; Li, Y. F.; Liu, J. D.; Wang, J. T.; He, Y. K.; Davey, K.; Qiao, S. Z. Molecular-level hybridization of Nafion with quantum dots for highly enhanced proton conduction. Adv. Mater. 2018, 30, 1707516.
Deng, W. Q.; Molinero, V.; Goddard, W. A. Fluorinated imidazoles as proton carriers for water-free fuel cell membranes. J. Am. Chem. Soc. 2004, 126, 15644–15645.
Choi, S. Y.; Cho, S.; Kim, D.; Kim, J.; Song, G.; Singh, R.; Kim, C. Boosting the proton conduction using protonated imidazole for advanced ion conducting membrane. J. Membr. Sci. 2021, 620, 118904.
Jalili, J.; Borsacchi, S.; Tricoli, V. Proton conducting membranes in fully anhydrous conditions at elevated temperature: Effect of Nitrilotris(methylenephosphonic acid) incorporation into Nafion-and poly(styrenesulfonic acid). J. Membr. Sci. 2014, 469, 162–173.
Di Noto, V.; Negro, E.; Sanchez, J. Y.; Iojoiu, C. Structure-relaxation interplay of a new nanostructured membrane based on tetraethylammonium trifluoromethanesulfonate ionic liquid and neutralized Nafion 117 for high-temperature fuel cells. J. Am. Chem. Soc. 2010, 132, 2183–2195.
Fu, Y. Z.; Manthiram, A. Nafion-imidazole-H3PO4 composite membranes for proton exchange membrane fuel cells. J. Electrochem. Soc. 2007, 154, B8–B12.
Yang, J. S.; Che, Q. T.; Zhou, L.; He, R. H.; Savinell, R. F. Studies of a high temperature proton exchange membrane based on incorporating an ionic liquid cation 1-butyl-3-methylimidazolium into a Nafion matrix. Electrochim. Acta 2011, 56, 5940–5946.
Lin, B. C.; Cheng, S.; Qiu, L. H.; Yan, F.; Shang, S. M.; Lu, J. M. Protic ionic liquid-based hybrid proton-conducting membranes for anhydrous proton exchange membrane application. Chem. Mater. 2010, 22, 1807–1813.
Meier, F.; Kerres, J.; Eigenberger, G. Methanol diffusion in water swollen ionomer membranes for DMFC applications. J. Membr. Sci. 2004, 241, 137–141.
Hallberg, F.; Vernersson, T.; Pettersson, E. T.; Dvinskikh, S. V.; Lindbergh, G.; Furó, I. Electrokinetic transport of water and methanol in Nafion membranes as observed by NMR spectroscopy. Electrochim. Acta 2010, 55, 3542–3549.
Chen, Z. W.; Holmberg, B.; Li, W. Z.; Wang, X.; Deng, W. Q.; Munoz, R.; Yan, Y. S. Nafion/zeolite nanocomposite membrane by in situ crystallization for a direct methanol fuel cell. Chem. Mater. 2006, 18, 5669–5675.
Chai, Z. L.; Wang, C.; Zhang, H. J.; Doherty, C. M.; Ladewig, B. P.; Hill, A. J.; Wang, H. T. Nafion-carbon nanocomposite membranes prepared using hydrothermal carbonization for proton-exchangemembrane fuel cells. Adv. Funct. Mater. 2010, 20, 4394–4399.
Li, J.; Fan, K.; Cai, W. W.; Ma, L. Y.; Xu, G. X.; Xu, S.; Ma, L.; Cheng, H. S. An in-situ nano-scale swelling-filling strategy to improve overall performance of Nafion membrane for direct methanol fuel cell application. J. Power Sources 2016, 332, 37–41.
Ladewig, B. P.; Knott, R. B.; Martin, D. J.; da Costa, J. C. D.; Lu, G. Q. Nafion-MPMDMS nanocomposite membranes with low methanol permeability. Electrochem. Commun. 2007, 9, 781–786.
Ladewig, B. P.; Knott, R. B.; Hill, A. J.; Riches, J. D.; White, J. W.; Martin, D. J.; Da Costa, J. C. D.; Lu, G. Q. Physical and electrochemical characterization of nanocomposite membranes of Nafion and functionalized silicon oxide. Chem. Mater. 2007, 19, 2372–2381.
Liu, B. L.; Hu, B.; Du, J.; Cheng, D. M.; Zang, H. Y.; Ge, X.; Tan, H. Q.; Wang, Y. H.; Duan, X. Z.; Jin, Z. et al. Precise molecular-level modification of Nafion with bismuth oxide clusters for high-performance proton-exchange membranes. Angew. Chem., Int. Ed. 2021, 133, 6141–6150.
Hanifpour, F.; Sveinbjörnsson, A.; Canales, C. P.; Skúlason, E.; Flosadóttir, H. D. Preparation of Nafion membranes for reproducible ammonia quantification in nitrogen reduction reaction experiments. Angew. Chem., Int. Ed. 2020, 59, 22938–22942.
Yin, C. S.; Li, J. J.; Zhou, Y. W.; Zhang, H. N.; Fang, P. F.; He, C. Q. Enhancement in proton conductivity and thermal stability in Nafion membranes induced by incorporation of sulfonated carbon nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035.
Xia, R.; Cao, X. Z.; Gao, M. Z.; Zhang, P.; Zeng, M. F.; Wang, B. Y.; Wei, L. Probing sub-nano level molecular packing and correlated positron annihilation characteristics of ionic cross-linked chitosan membranes using positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 3616–3626.
Wang, J. T.; Jiang, S.; Zhang, H.; Lv, W. J.; Yang, X. L.; Jiang, Z. Y. Enhancing proton conduction and methanol barrier performance of sulfonated poly(ether ether ketone) membrane by incorporated polymer carboxylic acid spheres. J. Membr. Sci. 2010, 364, 253–262.
Zhu, S. J.; Song, Y. B.; Shao, J. R.; Zhao, X. H.; Yang, B. Non-conjugated polymer dots with crosslink-enhanced emission in the absence of fluorophore units. Angew. Chem., Int. Ed. 2015, 54, 14626–14637.
Einsla, M. L.; Kim, Y. S.; Hawley, M.; Lee, H. S.; McGrath, J. E.; Liu, B. J.; Guiver, M. D.; Pivovar, B. S. Toward improved conductivity of sulfonated aromatic proton exchange membranes at low relative humidity. Chem. Mater. 2008, 20, 5636–5642.
Matos, B. R.; Dresch, M. A.; Santiago, E. I.; Moraes, L. P. R.; Carastan, D. J.; Schoenmaker, J.; Velasco-Davalos, I. A.; Ruediger, A.; Tavares, A. C.; Fonseca, F. C. Nafion membranes annealed at high temperature and controlled humidity: Structure, conductivity, and fuel cell performance. Electrochim. Acta 2016, 196, 110–117.
Matos, B. R.; Santiago, E. I.; Rey, J. F. Q.; Ferlauto, A. S.; Traversa, E.; Linardi, M.; Fonseca, F. C. Nafion-based composite electrolytes for proton exchange membrane fuel cells operating above 120 °C with titania nanoparticles and nanotubes as filler. J. Power Sources 2011, 196, 1061–1068.
Dimitrova, P.; Friedrich, K. A.; Stimming, U.; Vogt, B. Modified Nafion®-based membranes for use in direct methanol fuel cells. Solid State Ionics 2002, 150, 115–122.
Moore III, R. B.; Martin, C. R. Chemical and morphological properties of solution-cast perfluorosulfonate ionomers. Macromolecules 1988, 21, 1334–1339.
Choi, B. G.; Hong, J.; Park, Y. C.; Jung, D. H.; Hong, W. H.; Hammond, P. T.; Park, H. Innovative polymer nanocomposite electrolytes: Nanoscale manipulation of ion channels by functionalized graphenes. ACS Nano 2011, 5, 5167–5174.
Song, J.; Han, O. H.; Han, S. Nanometer-scale water- and proton-diffusion heterogeneities across water channels in polymer electrolyte membranes. Angew. Chem., Int. Ed. 2015 54, 3615–3620.
Yameen, B.; Kaltbeitzel, A.; Langer, A.; Müller, F.; Gösele, U.; Knoll, W.; Azzaroni, O. Highly proton-conducting self-humidifying microchannels generated by copolymer brushes on a scaffold. Angew. Chem., Int. Ed. 2009, 48, 3124–3128.
Yang, F.; Xu, G.; Dou, Y. B.; Wang, B.; Zhang, H.; Wu, H.; Zhou, W.; Li, J. R.; Chen, B. L. A flexible metal-organic framework with a high density of sulfonic acid sites for proton conduction. Nat. Energy 2017, 2, 877–883.
Yang, Y.; He, X. Y.; Zhang, P. H.; Andaloussi, Y. H.; Zhang, H. L.; Jiang, Z. Y.; Chen, Y.; Ma, S. Q.; Cheng, P.; Zhang, Z. J. Combined intrinsic and extrinsic proton conduction in robust covalent organic frameworks for hydrogen fuel cell applications. Angew. Chem., Int. Ed. 2020, 59, 3678–3684.
Peng, Q.; Li, Y.; Qiu, M.; Shi, B. B.; He, X. Y.; Fan, C. Y.; Mao, X. L.; Wu, H.; Jiang, Z. Y. Enhancing proton conductivity of sulfonated poly(ether ether ketone)-based membranes by incorporating phosphotungstic-acid-coupled graphene oxide. Ind. Eng. Chem. Res. 2021, 60, 4460–4470.
Acknowledgements
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (No. U2004199), the Excellent Youth Foundation of Henan Province (No. 202300410373), China Postdoctoral Science Foundation (Nos. 2021T140615 and 2020M672281), the Natural Science Foundation of Henan Province (No. 212300410285), and the Young Talent Support Project of Henan Province (No. 2021HYTP028). The center for advanced analysis and computational science, Zhengzhou University is also highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2022_4073_MOESM1_ESM.pdf
Manipulating the ionic nanophase of Nafion by in-situ precise hybridization with polymer quantum dot towards highly enhanced fuel cell performances
Rights and permissions
About this article
Cite this article
Wu, W., Zhou, Z., Wang, Y. et al. Manipulating the ionic nanophase of Nafion by in-situ precise hybridization with polymer quantum dot towards highly enhanced fuel cell performances. Nano Res. 15, 4124–4131 (2022). https://doi.org/10.1007/s12274-022-4073-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4073-4