Abstract
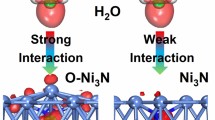
The high unoccupied d band energy of Ni3N basically results in weak orbital coupling with water molecule, consequently leading to slow water dissociation kinetics. Herein, we demonstrate Cr doping can downshift the unoccupied d orbitals and strengthen the interfacial orbital coupling to boost the water dissociation kinetics. The prepared Cr-Ni3N/Ni displays an impressive overpotential of 37 mV at 10 mA·cmgeo−2, close to the benchmark Pt/C in 1.0 M KOH solution. Refined structural analysis reveals the Cr dopant exists as the Cr-N6 states and the average d band energy of Ni3N is also lowered. Density functional theory calculation further confirms the downshifted d band energy can strengthen the orbital coupling between the unpaired electrons in O 2p and the unoccupied state of Ni 3d, which thus facilitates the water adsorption and dissociation. The work provides a new concept to achieve on-demand functions for hydrogen evolution catalysis and beyond, by regulating the interfacial orbital coupling.

Similar content being viewed by others
References
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
Zou, X. X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180.
Fei, H. L.; Dong, J. C.; Chen, D. L.; Hu, T. D.; Duan, X. D.; Shakir, I.; Huang, Y.; Duan, X. F. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241.
Niu, S.; Cai, J.; Wang G. Two-dimensional MOS2 for hydrogen evolution reaction catalysis: The electronic structure regulation. Nano Res. 2021, 14, 1985–2002.
Dresselhaus, M. S.; Thomas, I. L. Alternative energy technologies. Nature 2001, 414, 332–337.
Zhao, G. Q.; Rui, K.; Dou, S. X.; Sun, W. P. Heterostructures for electrochemical hydrogen evolution reaction: A review. Adv. Funct. Mater. 2018, 28, 1803291.
Gong M.; Wang D. Y.; Chen C. C.; Hwang B. J.; Dai H. J. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46.
Zhang, J. F.; Liu, C. B.; Zhang, B. Insights into single-atom metal-support interactions in electrocatalytic water splitting. Small Methods 2019, 3, 1800481.
Gong, M.; Zhou, D. W.; Kenney, M. J.; Kapusta, R.; Cowley, S.; Wu, D. Y. P.; Lu, D. B. G.; Lin, D. M. C.; Wang, D. D. Y.; Yang, D. J. et al. Blending Cr2O3 into a NiO-Ni electrocatalyst for sustained water splitting. Angew. Chem., Int. Ed. 2015, 54, 11989–11993.
Mahmood, J.; Li, F.; Jung, S. M.; Okyay, M. S.; Ahmad, I.; Kim, S. J.; Park, N.; Jeong, H. Y.; Baek, J. B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446.
Fei, H. L.; Dong, J. C.; Feng, Y. X.; Allen, C. S.; Wan, C. Z.; Volosskiy, B.; Li, M. F.; Zhao, Z. P.; Wang, Y. L.; Sun, H. T. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 2018, 1, 63–72.
Li, F.; Han, G. F.; Noh, H. J.; Ahmad, I.; Jeon, I. Y.; Baek, J. B. Mechanochemically assisted synthesis of a Ru catalyst for hydrogen evolution with performance superior to Pt in both acidic and alkaline media. Adv. Mater. 2018, 30, 1803676.
Wang, P. T.; Zhang, X.; Zhang, J.; Wan, S.; Guo, S. J.; Lu, G.; Yao, J. L.; Huang, X. Q. Precise tuning in platinum-nickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat. Commun. 2017, 8, 14580.
Wang, P. T.; Jiang, K. Z.; Wang, G. M.; Yao, J. L.; Huang, X. Q. Phase and interface engineering of platinum-nickel nanowires for efficient electrochemical hydrogen evolution. Angew. Chem., Int. Ed. 2016, 55, 12859–12863.
Lao, M. M.; Rui, K.; Zhao, G. Q.; Cui, P. X.; Zheng, X. S.; Dou, S. X.; Sun, W. P. Platinum/nickel bicarbonate heterostructures towards accelerated hydrogen evolution under alkaline conditions. Angew. Chem., Int. Ed. 2019, 58, 5432–5437.
Li, J. Y.; Liu, H. X.; Gou, W. Y.; Zhang, M. K.; Xia, Z. M.; Zhang, S.; Chang, C. R.; Ma, Y. Y.; Qu, Y. Q. Ethylene-glycol ligand environment facilitates highly efficient hydrogen evolution of Pt/CoP through proton concentration and hydrogen spillover. Energy Environ. Sci. 2019, 12, 2298–2304.
Deng, S. J.; Luo, M.; Ai, C. Z.; Zhang, Y.; Liu, B.; Huang, L.; Jiang, Z.; Zhang, Q. H.; Gu, L.; Lin, S. W. Synergistic doping and intercalation: Realizing deep phase modulation on MoS2 arrays for high-efficiency hydrogen evolution reaction. Angew. Chem., Int. Ed. 2019, 58, 16289–16296.
Kou, T. Y.; Smart, T.; Yao, B.; Chen, I.; Thota, D.; Ping, Y.; Li, Y. Theoretical and experimental insight into the effect of nitrogen doping on hydrogen evolution activity of Ni3S2 in alkaline medium. Adv. Energy Mater. 2018, 8, 1703538.
Chen, Z. Y.; Song, Y.; Cai, J. Y.; Zheng, X. S.; Han, D. D.; Wu, Y. S.; Zang, Y. P.; Niu, S. W.; Liu, Y.; Zhu, J. F. et al. Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis. Angew. Chem., Int. Ed. 2018, 57, 5076–5080.
Lv, Z.; Tahir, M.; Lang, X. W.; Yuan, G.; Pan, L.; Zhang, X. W.; Zou, J. J. Well-dispersed molybdenum nitrides on a nitrogen-doped carbon matrix for highly efficient hydrogen evolution in alkaline media. J. Mater. Chem. A 2017, 5, 20932–20937.
Gao, D. Q.; Zhang, J. Y.; Wang, T. T.; Xiao, W.; Tao, K.; Xue, D. S.; Ding, J. Metallic Ni3N nanosheets with exposed active surface sites for efficient hydrogen evolution. J. Mater. Chem. A 2016, 4, 17363–17369.
Liu, T. T.; Li, M.; Jiao, C. L.; Hassan, M.; Bo, X. J.; Zhou, M.; Wang, H. L. Design and synthesis of integrally structured Ni3N nanosheets/carbon microfibers/Ni3N nanosheets for efficient full water splitting catalysis. J. Mater. Chem. A 2017, 5, 9377–9390.
Fang, Y. Y.; Sun, D.; Niu, S. W.; Cai, J. Y.; Zang, Y. P.; Wu, Y. S.; Zhu, L. Q.; Xie, Y. F.; Liu, Y.; Zhu, Z. X. et al. Orbital-regulated interfacial electronic coupling endows Ni3N with superior catalytic surface for hydrogen evolution reaction. Sci. China Chem. 2020, 63, 1563–1569.
Yan, H. J.; Tian, C. G.; Wang, L.; Wu, A. P.; Meng, M. C.; Zhao, L.; Fu, H. G. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem., Int. Ed. 2015, 54, 6325–6329.
Wang, Y. Y.; Liu, D. D.; Liu, Z. J.; Xie, C.; Huo, J.; Wang, S. Y. Porous cobalt-iron nitride nanowires as excellent bifunctional electrocatalysts for overall water splitting. Chem. Commun. 2016, 52, 12614–12617.
Zhang, Y. Q.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R. S.; Fan, H. J. 3D porous hierarchical nickel-molybdenum nitrides synthesized by rf plasma as highly active and stable hydrogen-evolution-reaction electrocatalysts. Adv. Energy Mater. 2016, 6, 1600221.
Jia, X. D.; Zhao, Y. F.; Chen, G. B.; Shang, L.; Shi, R.; Kang, X. F.; Waterhouse, G. I. N.; Wu, L. Z.; Tung, C. H.; Zhang, T. R. Ni3FeN nanoparticles derived from ultrathin NiFe-layered double hydroxide nanosheets: An efficient overall water splitting electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585.
Shalom, M.; Ressnig, D.; Yang, X. F.; Clavel, G.; Fellinger, T. P.; Antonietti, M. Nickel nitride as an efficient electrocatalyst for water splitting. J. Mater. Chem. A 2015, 3, 8171–8177.
Liu, B.; He, B.; Peng, H. Q.; Zhao, Y. F.; Cheng, J. Y.; Xia, J.; Shen, J. H.; Ng, T. W.; Meng, X. M.; Lee, C. S. et al. Unconventional nickel nitride enriched with nitrogen vacancies as a high-efficiency electrocatalyst for hydrogen evolution. Adv. Sci. 2018, 5, 1800406.
Zhang, J.; Wang, T.; Liu, P.; Liao, Z. Q.; Liu, S. H.; Zhuang, X. D.; Chen, M. W.; Zschech, E.; Feng, X. L. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437.
Song, F. Z.; Li, W.; Yang, J. Q.; Han, G. Q.; Liao, P. L.; Sun, Y. J. Interfacing nickel nitride and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions. Nat. Commun. 2018, 9, 4531.
Wang, Y. H.; Chen, L.; Yu, X. M.; Wang, Y. G.; Zheng, G. F. Superb alkaline hydrogen evolution and simultaneous electricity generation by Pt-decorated Ni3N nanosheets. Adv. Energy Mater. 2017, 7, 1601390.
Niu, S. W.; Fang, Y. Y.; Zhou, J. B.; Cai, J. Y.; Zang, Y. P.; Wu, Y. S.; Ye, J.; Xie, Y. F.; Liu, Y.; Zheng, X. S. et al. Manipulating the water dissociation kinetics of Ni3N nanosheets via in situ interfacial engineering. J. Mater. Chem. A 2019, 7, 10924–10929.
Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K. C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260.
Zhu, C. R.; Wang, A. L.; Xiao, W.; Chao, D. L.; Zhang, X.; Tiep, N. H.; Chen, S.; Kang, J. N.; Wang, X.; Ding, J. et al. In situ grown epitaxial heterojunction exhibits high-performance electrocatalytic water splitting. Adv. Mater. 2018, 30, 1705516.
Xie, Y. F.; Cai, J. Y.; Wu, Y. S.; Zang, Y. P.; Zheng, X. S.; Ye, J.; Cui, P. X.; Niu, S. W.; Liu, Y.; Zhu, J. F. et al. Boosting water dissociation kinetics on Pt-Ni nanowires by N-induced orbital tuning. Adv. Mater. 2019, 31, 1807780.
Zang, Y. P.; Niu, S. W.; Wu, Y. S.; Zheng, X. S.; Cai, J. Y.; Ye, J.; Xie, Y. F.; Liu, Y.; Zhou, J. B.; Zhu, J. F. et al. Tuning orbital orientation endows molybdenum disulfide with exceptional alkaline hydrogen evolution capability. Nat. Commun. 2019, 10, 1217.
Yu H. S.; Wei X. J.; Li J.; Gu S. Q.; Zhang S.; Wang L. H.; Ma J. Y.; Li L. N.; Gao Q.; Si R., et al. The XAFS beamline of SSRF. Nucl. Sci. Tech. 2015, 26, 050102-1–050102-7.
McCrory, C. C. L.; Jung, S.; Ferrer, I. M.; Chatman, S. M.; Peters, J. C.; Jaramillo, T. F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357.
Wu, Y. S.; Liu, X. J.; Han, D. D.; Song, X. Y.; Shi, L.; Song, Y.; Niu, S. W.; Xie, Y. F.; Cai, J. Y.; Wu, S. Y. et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 2018, 9, 1425.
Clark, S. J.; Segall, M. D.; Pickard, C. J.; Hasnip, P. J.; Probert, M. J.; Refson, K.; Payne, M. C. First principles methods using castep. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865.
Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S. Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem., Int. Ed. 2015, 54, 52–65.
Wu, Y. Q.; Tao, X.; Qing, Y.; Xu, H.; Yang, F.; Luo, S.; Tian, C. H.; Liu, M.; Lu, X. H. Cr-doped FeNi-P nanoparticles encapsulated into N-doped carbon nanotube as a robust bifunctional catalyst for efficient overall water splitting. Adv. Mater. 2019, 31, 1900178.
Park, S. H.; Cho, Y. H.; Choi, M.; Choi, H.; Kang, J. S.; Um, J. H.; Choi, J. W.; Choe, H.; Sung, Y. E. Nickel-nitride-coated nickel foam as a counter electrode for dye-sensitized solar cells. Surf. Coat. Technol. 2014, 259, 560–569.
Wang, J. B.; Chen, W. L.; Wang, T.; Bate, N.; Wang, C. L.; Wang, E. B. A strategy for highly dispersed Mo2C/MoN hybrid nitrogen-doped graphene via ion-exchange resin synthesis for efficient electrocatalytic hydrogen reduction. Nano Res. 2018, 11, 4535–4548.
Ni, W. Y.; Krammer, A.; Hsu, C. S.; Chen, H. M.; Schüler, A.; Hu, X. L. Ni3N as an active hydrogen oxidation reaction catalyst in alkaline medium. Angew. Chem., Int. Ed. 2019, 58, 7445–7449.
Xu, S. Y.; Liu, C.; Kushwaha, S. K.; Sankar, R.; Krizan, J. W.; Belopolski, I.; Neupane, M.; Bian, G.; Alidoust, N.; Chang, T. R. et al. Observation of fermi arc surface states in a topological metal. Science 2015, 347, 294–298.
Deng, S. J.; Zhong, Y.; Zeng, Y. X.; Wang, Y. D.; Yao, Z. J.; Yang, F.; Lin, S. W.; Wang, X. L.; Lu, X. H.; Xia, X. H. et al. Directional construction of vertical nitrogen-doped 1T-2H MoSe2/graphene shell/core nanoflake arrays for efficient hydrogen evolution reaction. Adv. Mater. 2017, 29, 1700748.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Nos. 21771169 and 11722543), the National Key Research and Development Program of China (No. 2017YFA0206703), Anhui Provincial Natural Science Foundation (No. BJ2060190077), Collaborative Innovation Program of Hefei Science Center, CAS, and the Fundamental Research Funds for the Central Universities (Nos. WK2060190074, WK2060190081, WK2310000066, and WK2060000015). We also appreciate the Shanghai Synchrotron Radiation Facility (BL14W1, SSRF) and the Hefei National Synchrotron Radiation Laboratory (BL10B, NSRL) for the help in XAFS, UPS and XPS characterizations. The computational research in this paper has been done on the supercomputing system in the Supercomputing Center of University of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wu, Y., Xie, Y., Niu, S. et al. Accelerating water dissociation kinetics of Ni3N by tuning interfacial orbital coupling. Nano Res. 14, 3458–3465 (2021). https://doi.org/10.1007/s12274-021-3562-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3562-1