Abstract
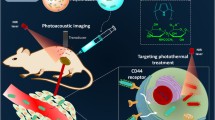
As a new type of cancer treatment, photoacoustic (PA) therapy is based on PA shockwave for rapid, selective and effective killing of cancer cells. The nucleus has been widely used as a target for tumor therapy, which has obtained a very considerable therapeutic effect. In situ destruction of tumor cell nucleus by photoacoustic therapy has not been studied. In this paper, a highly efficient nucleus-targeted photoacoustic theranostic polymer was developed for fluorescence and photoacoustic dual-mode imaging-guided PA therapy. The prepared polymer consists of nucleus targeting TAT peptide (TAT: YGRKKRRQRRR), hydrophilic chain poly (N,N-dimethylacrylamide) (PDMA), and near-infrared (NIR) light absorbing agent (hCyR), which can self-assemble to form nanoparticles of approximately 28 nm (denoted as TAT-PDMA-hCyR NPs). The designed nanoparticles show excellent nucleus targeting and tumor cell death (up to 80%) caused by DNA damage under pulsed laser irradiation compared to non-nucleus target counterpart PDMA-hCyR NPs without TAT peptide in vitro. As expected, the fluorescence and PA dual-mode imaging observed that TAT-PDMA-hCyR NPs were able to passively target and enrich in tumors, providing an experimental basis for in vivo treatment and thus ensuring a significant tumor inhibition rate (about 92%). In conclusion, this study provides a new and practicable method for the development of nucleus-targeting nanoparticles as potential theranostic agent for in vivo cancer imaging and therapy.

Similar content being viewed by others
References
Torre, L. A.; Siegel, R. L.; Ward, E. M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol., Biomarkers Prev.2016, 25, 16–27.
Siegel, R. L.; Miller, K. D.; Jemal, A. Cancer statistics, 2016. CA: Cancer J. Clin.2016, 66, 7–30.
Archer, S. L. Mitochondrial dynamics-mitochondrial fission and fusion in human diseases. N. Engl. J. Med.2013, 369, 2236–2251.
Arnoult, D. Mitochondrial fragmentation in apoptosis. Trends Cell. Biol.2007, 17, 6–12.
Zhang, X.; Ba, Q.; Gu, Z. N.; Guo, D. L.; Zhou, Y.; Xu, Y. G.; Wang, H.; Ye, D. J.; Liu, H. Fluorescent coumarin-artemisinin conjugates as mitochondria-targeting theranostic probes for enhanced anticancer activities. Chemistry2015, 21, 17415–17421.
Dai, L. L.; Cai, R. S.; Li, M. H.; Luo, Z.; Yu, Y. L.; Chen, W. Z.; Shen, X. K.; Pei, Y. X.; Zhao, X. J.; Cai, K. Y. Dual-targeted cascade-responsive prodrug micelle system for tumor therapy in vivo.Chem. Mater.2017, 29, 6976–6992.
de la Fuente, J. M.; Berry, C. C. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjugate Chem.2005, 16, 1176–1180.
Patel, S. S.; Belmont, B. J.; Sante, J. M.; Rexach, M. F. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell2007, 129, 83–96.
Alber, F.; Dokudovskaya, S.; Veenhoff, L. M.; Zhang, W. Z.; Kipper, J.; Devos, D.; Suprapto, A.; Karni-Schmidt, O.; Williams, R.; Chait, B. T. et al. The molecular architecture of the nuclear pore complex. Nature2007, 450, 695–701.
van der Aa, M. A. E. M.; Mastrobattista, E.; Oosting, R. S.; Hennink, W. E.; Koning, G. A.; Crommelin, D. J. A. The nuclear pore complex: The gateway to successful nonviral gene delivery. Pharm. Res.2006, 23, 447–459.
Kubitscheck, U.; Grünwald, D.; Hoekstra, A.; Rohleder, D.; Kues, T.; Siebrasse, J. P.; Peters, R. Nuclear transport of single molecules: Dwell times at the nuclear pore complex. J. Cell. Biol.2005, 168, 233–243.
Kosugi, S.; Hasebe, M.; Entani, T.; Takayama, S.; Tomita, M.; Yanagawa, H. Design of peptide inhibitors for the importin α/β nuclear import pathway by activity-based profiling. Chem. Biol.2008, 15, 940–949.
Nitin, N.; LaConte, L.; Rhee, W. J.; Bao, G. Tat peptide is capable of importing large nanoparticles across nuclear membrane in digitonin permeabilized cells. Ann. Biomed. Eng.2009, 37, 2018–2027.
Tkachenko, A. G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M. F.; Franzen, S.; Feldheim, D. L. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc.2003, 125, 4700–4701.
Austin, L. A.; Kang, B.; Yen, C. W.; El-Sayed, M. A. Nuclear targeted silver nanospheres perturb the cancer cell cycle differently than those of nanogold. Bioconjugate Chem.2011, 22, 2324–2331.
Austin, L. A.; Kang, B.; Yen, C. W.; El-Sayed, M. A. Plasmonic imaging of human oral cancer cell communities during programmed cell death by nuclear-targeting silver nanoparticles. J. Am. Chem. Soc.2011, 133, 17594–17597.
Pan, L. M.; Liu, J. N.; Shi, J. L. Intranuclear photosensitizer delivery and photosensitization for enhanced photodynamic therapy with ultralow irradiance. Adv. Funct. Mater.2014, 24, 7318–7327.
Pan, L. M.; Liu, J. N.; He, Q. J.; Shi, J. L. MSN-mediated sequential vascular-to-cell nuclear-targeted drug delivery for efficient tumor regression. Adv. Mater.2014, 26, 6742–6748.
Pan, L. M.; He, Q. J.; Liu, J. N.; Chen, Y.; Ma, M.; Zhang, L. L.; Shi, J. L. Nuclear-targeted drug delivery of tat peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc.2012, 134, 5722–5725.
Tan, L. F.; Tang, W. T.; Liu, T. L.; Ren, X. L.; Fu, C. H.; Liu, B.; Ren, J.; Meng, X. W. Biocompatible hollow polydopamine nanoparticles loaded ionic liquid enhanced tumor microwave thermal ablation in vivo.ACS Appl. Mater. Interfaces2016, 8, 11237–11245.
Park, N. H.; Cheng, W.; Lai, F.; Yang, C.; Florez de Sessions, P.; Periaswamy, B.; Wenhan Chu, C.; Bianco, S.; Liu, S. Q.; Venkataraman, S. et al. Addressing drug resistance in cancer with macromolecular chemotherapeutic agents. J. Am. Chem. Soc.2018, 140, 4244–4252.
Zang, Y. D.; Wei, Y. C.; Shi, Y. J.; Chen, Q.; Xing, D. Chemo/photoacoustic dual therapy with mRNA-triggered dox release and photoinduced shockwave based on a DNA-gold nanoplatform. Small2016, 12, 756–769.
Kang, B.; Yu, D. C.; Dai, Y. D.; Chang, S. Q.; Chen, D.; Ding, Y. T. Cancer-cell targeting and photoacoustic therapy using carbon nanotubes as “bomb” agents. Small2009, 5, 1292–1301.
Huang, G. J.; Si, Z.; Yang, S. H.; Li, C.; Xing, D. Dextran based pH-sensitive near-infrared nanoprobe for in vivo differential-absorption dual-wavelength photoacoustic imaging of tumors. J. Mater. Chem.2012, 22, 22575–22581.
Wen, L. W.; Ding, W. Z.; Yang, S. H.; Xing, D. Microwave pumped high-efficient thermoacoustic tumor therapy with single wall carbon nanotubes. Biomaterials2016, 75, 163–173.
Zhong, J. P.; Wen, L. W.; Yang, S. H.; Xiang, L. Z.; Chen, Q.; Xing, D. Imaging-guided high-efficient photoacoustic tumor therapy with targeting gold nanorods. Nanomedicine2015, 11, 1499–1509.
Zhou, F. F.; Wu, S. N.; Yuan, Y.; Chen, W. R.; Xing, D. Mitochondria-targeting photoacoustic therapy using single-walled carbon nanotubes. Small2012, 8, 1543–1550.
Kang, B.; Dai, Y. D.; Chang, S. Q.; Chen, D. Explosion of singlewalled carbon nanotubes in suspension induced by a large photo-acoustic effect. Carbon2008, 46, 978–981.
Shi, Y. J.; Yang, S. H.; Xing, D. New insight into photoacoustic conversion efficiency by plasmon-mediated nanocavitation: Implications for precision theranostics. Nano Res.2017, 10, 2800–2809.
Chen, A. P.; Xu, C.; Li, M.; Zhang, H. L.; Wang, D. C.; Xia, M.; Meng, G.; Kang, B.; Chen, H. Y.; Wei, J. W. Photoacoustic “nanobombs” fight against undesirable vesicular compartmentalization of anticancer drugs. Sci. Rep.2015, 5, 15527.
Liu, L. M.; Chen, Q.; Wen, L. W.; Li, C.; Qin, H.; Xing, D. Photoacoustic therapy for precise eradication of glioblastoma with a tumor site blood-brain barrier permeability upregulating nanoparticle. Adv. Funct. Mater.2019, 29, 1808601.
Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev.2018, 118, 6844–6892.
Gupta, M. K.; Meyer, T. A.; Nelson, C. E.; Duvall, C. L. Poly(PS-b-DMA) micelles for reactive oxygen species triggered drug release. J. Controlled Release2012, 162, 591–598.
Bhattacharya, A.; Mukherjee, T. K. Synergistic enhancement of electron-accepting and -donating ability of nonconjugated polymer nanodot in micellar environment. Langmuir2017, 33, 14718- 14727.
Cheng, Y.; Sun, C. L.; Ou, X. W.; Liu, B. F.; Lou, X. D.; Xia, F. Dual-targeted peptide-conjugated multifunctional fluorescent probe with aiegen for efficient nucleus-specific imaging and long-term tracing of cancer cells. Chem. Sci.2017, 8, 4571–4578.
Toy, R.; Bauer, L.; Hoimes, C.; Ghaghada, K. B.; Karathanasis, E. Targeted nanotechnology for cancer imaging. Adv. Drug Deliv. Rev.2014, 76, 79–97.
Pan, J. B.; Wang, Y. Q.; Zhang, C.; Wang, X. Y.; Wang, H. Y.; Wang, J. J.; Yuan, Y. Z.; Wang, X.; Zhang, X. J.; Yu, C. S. et al. Antigen-directed fabrication of a multifunctional nanovaccine with ultrahigh antigen loading efficiency for tumor photothermal-immunotherapy. Adv. Mater.2018, 30, 1704408.
Cai, Y. B.; Shen, H. S.; Zhan, J.; Lin, M. L.; Dai, L. H.; Ren, C. H.; Shi, Y.; Liu, J. F.; Gao, J.; Yang, Z. M. Supramolecular “trojan horse” for nuclear delivery of dual anticancer drugs. J. Am. Chem. Soc.2017, 139, 2876–2879.
Yuan, L.; Lin, W. Y.; Yang, Y. T.; Chen, H. A unique class of near-infrared functional fluorescent dyes with carboxylic-acid-modulated fluorescence on/off switching: Rational design, synthesis, optical properties, theoretical calculations, and applications for fluorescence imaging in living animals. J. Am. Chem. Soc.2012, 134, 1200–1211.
Hu, X. L.; Liu, G. H.; Li, Y.; Wang, X. R.; Liu, S. Y. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J. Am. Chem. Soc.2015, 137, 362–368.
Qiu, X. P.; Winnik, F. M. Facile and efficient one-pot transformation of RAFT polymer end groups via a mild aminolysis/michael addition sequence. Macromol. Rapid Commun.2006, 27, 1648–1653.
Peng, H. B.; Tang, J.; Zheng, R.; Guo, G. N.; Dong, A. A.; Wang, Y. J.; Yang, W. L. Nuclear-targeted multifunctional magnetic nanoparticles for photothermal therapy. Adv. Healthc. Mater.2017, 6, 1601289.
Hu, X. L.; Zhai, S. D.; Liu, G. H.; Xing, D.; Liang, H. L. Liu, S. Y. Concurrent drug unplugging and permeabilization of polyprodrug-gated crosslinked vesicles for cancer combination chemotherapy. Adv. Mater.2018, 30, 1706307.
Zhou, F. F.; Wu, S. N.; Song, S.; Chen, W. R.; Resasco, D. E.; Xing, D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials2012, 33, 3235–3242.
Zhao, N.; Wu, B. Y.; Hu, X. L.; Xing, D. Nir-triggered high-efficient photodynamic and chemo-cascade therapy using caspase-3 responsive functionalized upconversion nanoparticles. Biomaterials2017, 141, 40–49.
Roth, P. J.; Boyer, C.; Lowe, A. B.; Davis, T. P. Raft polymerization and thiol chemistry: A complementary pairing for implementing modern macromolecular design. Macromol. Rapid Commun.2011, 32, 1123–1143.
Roy, E.; Patra, S.; Madhuri, R.; Sharma, P. K. RETRACTED: Carbon dot/tat peptide co-conjugated bubble nanoliposome for multicolor cell imaging, nuclear-targeted delivery, and chemo/photothermal synergistic therapy. Chem. Eng. J.2017, 312, 144–157.
Hu, X. L.; Li, Y.; Liu.; Zhang, G. Y.; Liu, S. Y. Intracellular cascade fret for temperature imaging of living cells with polymeric ratiometric fluorescent thermometers. ACS Appl. Mater. Interfaces2015, 7, 15551–15560.
Vankayala, R.; Kuo, C. L.; Nuthalapati, K.; Chiang, C. S.; Hwang, K. C. Nucleus-targeting gold nanoclusters for simultaneous in vivo fluorescence imaging, gene delivery, and NIR-light activated photodynamic therapy. Adv. Funct. Mater.2015, 25, 5934–5945.
Zhai, S. D.; Hu, X. L.; Hu, Y. J.; Wu, B. Y.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials2017, 121, 41–54.
Chen, Q.; Liu, X. D.; Chen, J. W.; Zeng, J. F.; Cheng, Z. P.; Liu, Z. A self-assembled albumin-based nanoprobe for in vivo ratiometric photoacoustic pH imaging. Adv. Mater.2015, 27, 6820–6827.
Zhang, S. B.; Guo, W. S.; Wei, J.; Li, C.; Liang, X. J.; Yin, M. Z. Terrylenediimide-based intrinsic theranostic nanomedicines with high photothermal conversion efficiency for photoacoustic imaging-guided cancer therapy. Acs Nano2017, 11, 3797–3805.
Yang, C. H.; Ren, C. H.; Zhou, J.; Liu, J. J.; Zhang, Y. M.; Huang, F.; Ding, D.; Xu, B.; Liu, J. F. Dual fluorescent- and isotopic-labelled self-assembling vancomycin for in vivo imaging of bacterial infections. Angew. Chem., Int. Ed.2017, 56, 2356–2360.
Li, J. W.; Xiao, H.; Yoon, S. J.; Liu, C. B.; Matsuura, D.; Tai, W. Y.; Song, L.; O’Donnell, M.; Cheng, D.; Gao, X. H. Functional photo-acoustic imaging of gastric acid secretion using pH-responsive polyaniline nanoprobes. Small2016, 12, 4690–4696.
Guo, L.; Niu, G. L.; Zheng, X. L.; Ge, J. C.; Liu, W. M.; Jia, Q. Y.; Zhang, P. P.; Zhang, H. Y.; Wang, P. F. Single near-infrared emissive polymer nanoparticles as versatile phototheranostics. Adv. Sci.2017, 4, 1700085.
Chen, Q.; Liu, X. D.; Zeng, J. F.; Cheng, Z. P.; Liu, Z. Albumin-NIR dye self-assembled nanoparticles for photoacoustic pH imaging and pH-responsive photothermal therapy effective for large tumors. Biomaterials2016, 98, 23–30.
Wang, D.; Lee, M.; Xu, W.; Shan, G.; Zheng, X.; Kwok, R.; Lam, J.; Hu, X.; Tang, B. Boosting non-radiative decay to do useful work: Development of a multi-modality theranostic system from an AIEgen. Angew. Chem., Int. Ed.2019, 58, 5628–5632.
Jin, E. L.; Zhang, B.; Sun, X. R.; Zhou, Z. X.; Ma, X. P.; Sun, Q. H.; Tang, J. B.; Shen, Y. Q.; Van Kirk, E.; Murdoch, W. J. et al. Acid-active cell-penetrating peptides for in vivo tumor-targeted drug delivery. J. Am. Chem. Soc.2013, 135, 933–940.
Guan, M. R.; Ge, J. C.; Wu, J. Y.; Zhang, G. Q.; Chen, D. Q.; Zhang, W.; Zhang, Y.; Zou, T. J.; Zhen, M. M.; Wang, C. R. et al. Fullerene/photosensitizer nanovesicles as highly efficient and clearable photo-theranostics with enhanced tumor accumulation for cancer therapy. Biomaterials2016, 103, 75–85.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81630046) and the Science and Technology Planning Project of Guangdong Province (Nos. 2015B020233016 and 2014B020215003)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wang, Y., Niu, G., Zhai, S. et al. Specific photoacoustic cavitation through nucleus targeted nanoparticles for high-efficiency tumor therapy. Nano Res. 13, 719–728 (2020). https://doi.org/10.1007/s12274-020-2681-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2681-4