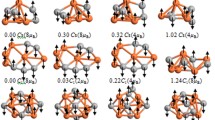
Abstract
Superexchange effects play an important role in the determination of crystal structures; however, there has been much less reported on how they determine the stability of clusters. Using evolutionary search strategies and DFT+U (density functional theory with the Hubbard U correction) calculations, we investigate the global minimum-energy structures of Fe12On clusters. Among predicted Fe12On clusters, a cage-shaped Fe12O12 cluster with unexpected stability was observed. In addition, the bare Fe12O12 cluster is shown to possess an extremely large energy gap (2.00 eV), which is greater than that of C60, Au20 and Al13−clusters. Using a Heisenberg model, we traced the origin of the unexpected stability of the bare Fe12O12 cluster to magnetic competition between the nearest-neighbor exchange constant J1 and the next-nearest neighbor exchange constant J2 that was induced by the superexchange interactions. The bare Fe12O12 cluster is thus a unique molecule that is stable and chemically inert.

Similar content being viewed by others
References
Khanna, S. N.; Jena, P. Assembling crystals from clusters. Phys. Rev. Lett. 1992, 69, 1664–1667.
Chang, C.-R.; Huang, Z.-Q.; Li, J. Hydrogenation of molecular oxygen to hydroperoxyl: An alternative pathway for O2 activation on nanogold catalysts. Nano Res. 2015, 8, 3737–3748.
Lv, C. L.; Cheng, H.; He, W.; Shah, M. I. A.; Xu, C. Q.; Meng, X. J.; Jiao, L.; Wei, S. Q.; Li, J.; Liu, L. et al. Pd3 cluster catalysis: Compelling evidence from in operando spectroscopic, kinetic, and density functional theory studies. Nano Res. 2016, 9, 2544–2550.
Qiao, B.; Liang, J.-X.; Wang, A. Q.; Xu, C.-Q.; Li, J.; Zhang, T.; Liu, J. J. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI). Nano Res. 2015, 8, 2913–2924.
Long, B.; Tang, Y.; Li, J. New mechanistic pathways for CO oxidation catalyzed by single-atom catalysts: Supported and doped Au1/ThO2. Nano Res. 2016, 9, 3868–3880.
Echt, O.; Sattler, K.; Recknagel, E. Magic numbers for sphere packings: Experimental verification in free xenon clusters. Phys. Rev. Lett. 1981, 47, 1121–1124.
Knight, W.-D.; Clemenger, K.; de Heer, W. A.; Saunders, W. A.; Chou, M. Y.; Cohen, M. L. Electronic shell structure and abundances of sodium clusters. Phys. Rev. Lett. 1984, 52, 2141–2143.
Reveles, J. U.; Clayborne, P. A.; Reber, A. C.; Khanna, S. N.; Pradhan, K.; Sen, P.; Pederson, M. R. Designer magnetic superatoms. Nat. Chem. 2009, 1, 310–315.
Li, X.; Kuznetsov, A. E.; Zhang, H. F.; Boldyrev, A. I.; Wang, L. S. Observation of all-metal aromatic molecules. Science 2001, 291, 859–861.
Yu, X. H.; Oganov, A. R.; Popov, I. A.; Boldyrev, A. I. d-AO spherical aromaticity in Ce6O8. J. Comput. Chem. 2016, 37, 103–109.
Yu, X. H.; Oganov, A. R.; Popov, I. A.; Qian, G. R.; Boldyrev, A. I. Antiferromagnetic stabilization in the Ti8O12 cluster. Angew. Chem., Int. Ed. 2016, 55, 1699–1703.
Shiroishi, H.; Oda, T.; Hamada, I.; Fujima, N. Structure and magnetism on iron oxide clusters FenOm (n = 1–5): Calculation from first principles. Eur. Phys. J. D: Atomic, Mol. Opt. Phys. 2003, 24, 85–88.
Wu, H. B.; Desai, S. R.; Wang, L.-S. Observation and photoelectron spectroscopic study of novel mono- and diiron oxide molecules: FeOy − (y = 1−4) and Fe2Oy − (y = 1−5). J. Am. Chem. Soc. 1996, 118, 5296–5301.
Wang, L.-S.; Wu, H. B.; Desai, S. R. Sequential oxygen atom chemisorption on surfaces of small iron clusters. Phys. Rev. Lett. 1996, 76, 4853–4856.
Kirilyuk, A.; Fielicke, A.; Demyk, K.; von Helden, G.; Meijer, G.; Rasing, T. Ferrimagnetic cagelike Fe4O6 cluster: Structure determination from infrared dissociation spectroscopy. Phys. Rev. B 2010, 82, 020405.
Reddy, B. V.; Khanna, S. N. Self-stimulated NO reduction and CO oxidation by iron oxide clusters. Phys. Rev. Lett. 2004, 93, 068301.
Gutsev, G. L.; Khanna, S. N.; Rao, B. K.; Jena, P. FeO4: A unique example of a closed-shell cluster mimicking a superhalogen. Phys. Rev. A 1999, 59, 3681–3684.
Jones, N. O.; Reddy, B. V.; Rasouli, F.; Khanna, S. N. Structural growth in iron oxide clusters: Rings, towers, and hollow drums. Phys. Rev. B 2005, 72, 165411.
Ding, X.-L.; Xue, W.; Ma, Y.-P.; Wang, Z.-C.; He, S.-G. Density functional study on cage and noncage (Fe2O3)n clusters. J. Chem. Phys. 2009, 130, 014303.
Erlebach, A.; Hühn, C.; Jana, R.; Sierka, M. Structure and magnetic properties of (Fe2O3)n clusters (n = 1–5). Phys. Chem. Chem. Phys. 2014, 16, 26421–26426.
Gutsev, G. L.; Weatherford, C. A.; Jena, P.; Johnson, E.; Ramachandran, B. R. Competition between surface chemisorption and cage formation in Fe12O12 clusters. Chem. Phys. Lett. 2013, 556, 211–216.
Mejía-López, A.; Mazo-Zuluaga, J.; Mejía-López, J. Sequential oxygen chemisorption on Fe13 clusters: From first-principles to practical insights. J. Phys.:Condens. Matter 2016, 28, 485002.
Palotás, K.; Andriotis, A. N.; Lappas, A. Structural, electronic, and magnetic properties of nanometer-sized iron-oxide atomic clusters: Comparison between GGA and GGA+U approaches. Phys. Rev. B 2010, 81, 075403.
Oganov, A. R.; Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J. Chem. Phys. 2006, 124, 244704.
Zhang, W. W.; Oganov, A. R.; Goncharov, A. F.; Zhu, Q.; Boulfelfel, S. E.; Lyakhov, A. O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V. B.; Konôpková, Z. Unexpected stable stoichiometries of sodium chlorides. Science 2013, 342, 1502–1505.
Zhou, X.-F.; Dong, X.; Oganov, A. R.; Zhu, Q.; Tian, Y. J.; Wang, H.-T. Semimetallic two-dimensional boron allotrope with massless dirac fermions. Phys. Rev. Lett. 2014, 112, 085502.
Oganov, A. R.; Lyakhov, A. O.; Valle, M. How evolutionary crystal structure prediction works—and why. Acc. Chem. Res. 2011, 44, 227–237.
Lyakhov, A. O.; Oganov, A. R.; Stokes, H. T.; Zhu, Q. New developments in evolutionary structure prediction algorithm uspex. Comput. Phys. Commun. 2013, 184, 1172–1182.
Zhu, Q.; Sharma, V.; Oganov, A. R.; Ramprasad, R. Predicting polymeric crystal structures by evolutionary algorithms. J. Chem. Phys. 2014, 141, 154102.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Dudarev, S. L.; Botton, G. A.; Savrasov, S. Y.; Humphreys, C. J.; Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509.
Yu, X. H.; Wang, S.-G.; Li, Y.-W.; Wang, J. G.; Jiao, H. J. Single gold atom adsorption on the Fe3O4(111) surface. J. Phys. Chem. C 2012, 116, 10632–10638.
Yu, X. H.; Huo, C.-F.; Li, Y.-W.; Wang, J. G.; Jiao, H. J. Fe3O4 surface electronic structures and stability from GGA+U. Surf. Sci. 2012, 606, 872–879.
Kulik, H. J.; Cococcioni, M.; Scherlis, D. A.; Marzari, N. Density functional theory in transition-metal chemistry: A self-consistent Hubbard U approach. Phys. Rev. Lett. 2006, 97, 103001.
Yu, X. H.; Zhang, X. M.; Jin, L. X.; Feng, G. CO adsorption, oxidation and carbonate formation mechanisms on Fe3O4 surfaces. Phys. Chem. Chem. Phys. 2017, 19, 17287–17299.
Yu, X. H.; Zhang, X. M.; Wang, S. G.; Feng, G. Adsorption of Aun (n = 1–4) clusters on Fe3O4(001) B-termination. RSC Adv. 2015, 5, 45446–45453.
Yu, X. H.; Zhang, X. M.; Wang, S. G. High coverage hydrogen adsorption on the Fe3O4(110) surface. Appl. Surf. Sci. 2015, 353, 973–978.
Bhattacharya, S.; Levchenko, S. V.; Ghiringhelli, L. M.; Scheffler, M. Stability and metastability of clusters in a reactive atmosphere: Theoretical evidence for unexpected stoichiometries of MgmOx. Phys. Rev. Lett. 2013, 111, 135501.
Yu, X. H.; Zhang, X. M.; Wang, H. T.; Wang, Z. Y.; Feng, G. High-coverage H2 adsorption on the reconstructed Cu2O(111) surface. J. Phys. Chem. C 2017, 121, 22081–22091.
Yu, X. H.; Zhang, X. M. High coverage water adsorption on CuO(011) surface. Phys. Chem. Chem. Phys. 2017, 19, 18652–18659.
Yu, X. H.; Zhang, X. M.; Wang, S. G.; Feng, G. A computational study on water adsorption on Cu2O(111) surfaces: The effects of coverage and oxygen defect. Appl. Surf. Sci. 2015, 343, 33–40.
Stull, D. R.; Prophet, H. Janaf thermochemical tables; U. S. EPO: Washington, DC, 1971.
Yu, X. H.; Zhang, X. M.; Wang, H. T.; Feng, G. High coverage water adsorption on the CuO(111) surface. Appl. Surf. Sci. 2017, 425, 803–810.
de Oliveira, O. V.; Pires, J. M.; Neto, A. C.; Divino dos Santos, J. Computational studies of the Ca12O12, Ti12O12, Fe12O12 and Zn12O12 nanocage clusters. Chem. Phys. Lett. 2015, 634, 25–28.
Gong, X. G.; Kumar, V. Enhanced stability of magic clusters: A case study of icosahedral Al12X, X=B, Al, Ga, C, Si, Ge, Ti, As. Phys. Rev. Lett. 1993, 70, 2078–2081.
Bergeron, D. E.; Castleman, A. W.; Morisato, T.; Khanna, S. N. Formation of Al13I–: Evidence for the superhalogen character of Al13. Science 2004, 304, 84–87.
Li, J.; Li, X.; Zhai, H.-J.; Wang, L.-S. Au20: A tetrahedral cluster. Science 2003, 299, 864–867.
Wang, X. B.; Ding, C. F.; Wang, L. S. High resolution photoelectron spectroscopy of C60 −. J. Chem. Phys. 1999, 110, 8217–8220.
Kroto, H. W.; Heath, J. R.; O’Brien, S. C.; Curl, R. F.; Smalley, R. E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11474004), the National Science Foundation of Henan Province (No. 162300410001) and the Natural Science Foundation of Shaanxi University of Technology (No. SLGQD2017-13). Calculations were performed on Rurik supercomputer at Moscow Institute of Physics and Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yu, X., Zhang, X. & Yan, XW. Stability of the Fe12O12 cluster. Nano Res. 11, 3574–3581 (2018). https://doi.org/10.1007/s12274-017-1923-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1923-6