Abstract
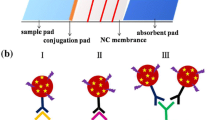
For rapid and simultaneous detection of (fluoro)quinolones, a broadly specific monoclonal antibody (mAb) that recognizes 32 (fluoro)quinolone antibiotics was prepared using a mixture of a norfloxacin derivative and a sarfloxacin derivative as the hapten. An immunochromatographic strip based on gold nanoparticles (AuNPs) was then assembled with goat anti-mouse antibody and antigen (sarfloxacin coupled to ovalbumin), used to form the C line and T line, respectively. This antigen competes with the (fluoro)quinolones in a sample incubated with mAbs labeled with AuNPs. The strip can detect 32 (fluoro)quinolones including oxolinic acid, nalidixic acid, miloxacin, pipemidic acid, piromidic acid, rosoxacin, cinoxacin, norfloxacin, pefloxacin, lomfloxacin, enofloxacin, fleroxacin, ciprofloxacin, enrofloxacin, dafloxacin, orbifloxacin, sparfloxacin, gemifloxacin, besifloxacin, balofloxacin, gatifloxacin, moxifloxacin, nadifloxacin, ofloxacin, marbofloxacin, flumequine, pazufloxacin, prulifloxacin, sarafloxacin, difloxacin, trovafloxacin, and tosufloxacin in milk within 10 min with the naked eye. The cut-off values of the strip range from 1 to 100 ng/mL and the limits of detection are 0.1–10 ng/mL. The strip does not cross-react with antibiotics including tetracycline, sulfamethazine, ampicillin, erythromycin, aflatoxin B1, or gentamicin. In short, this immunochromatographic strip is a very useful tool for the primary screening of (fluoro)quinolones in milk.

Similar content being viewed by others
References
Ceyssens, P.-J.; Van Bambeke, F.; Mattheus, W.; Bertrand, S.; Fux, F.; Van Bossuyt, E.; Damée, S.; Nyssen, H.-J.; De Craeye, S.; Verhaegen, J. et al. Molecular analysis of rising fluoroquinolone resistance in Belgian non-invasive streptococcus pneumoniae isolates (1995–2014). PLoS One 2016, 11, e0154816.
Suryoprabowo, S.; Liu, L. Q.; Peng, J.; Kuang, H.; Xu, C. L. Development of a broad specific monoclonal antibody for fluoroquinolone analysis. Food Anal. Meth. 2014, 7, 2163–2168.
Takahashi, H.; Hayakawa, I.; Akimoto, T. The history of the development and changes of quinolone antibacterial agents. Yakushigaku Zasshi 2003, 38, 161–179.
Zhu, Y.; Li, L.; Wang, Z. H.; Chen, Y. Q.; Zhao, Z. M.; Zhu, L.; Wu, X. P.; Wan, Y. P.; He, F. Y.; Shen, J. Z. Development of an immunochromatography strip for the rapid detection of 12 fluoroquinolones in chicken muscle and liver. J. Agric. Food Chem. 2008, 56, 5469–5474.
Rubinstein, E. History of quinolones and their side effects. Chemotherapy 2001, 47, 3–8.
Wu, X.-L.; Xiang, L.; Yan, Q.-Y.; Jiang, Y.-N.; Li, Y.-W.; Huang, X.-P.; Li, H.; Cai, Q.-Y.; Mo, C.-H. Distribution and risk assessment of quinolone antibiotics in the soils from organic vegetable farms of a subtropical city, Southern China. Sci. Total Environ. 2014, 487, 399–406.
Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.-F.; Larsson, D. G. J. Fluoroquinolones and qnr genes in sediment, water, soil, and human fecal flora in an environment polluted by manufacturing discharges. Environ. Sci. Technol. 2014, 48, 7825–7832.
Wang, H. X.; Wang, B.; Zhao, Q.; Zhao, Y. P.; Fu, C. W.; Feng, X.; Wang, N.; Su, M. F.; Tang, C. X.; Jiang, F. et al. Antibiotic body burden of Chinese school children: A multisite biomonitoring-based study. Environ. Sci. Technol. 2015, 49, 5070–5079.
Wang, H. X.; Wang, N.; Wang, B.; Fang, H.; Fu, C. W.; Tang, C. X.; Jiang, F.; Zhou, Y.; He, G. S.; Zhao, Q. et al. Antibiotics detected in urines and adipogenesis in school children. Environ. Int. 2016, 89–90, 204–211.
Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme; Rome, Italy, 2012.
The Ministry of Agriculture. Gazette of the Ministry of Agriculture of The People’s Republic of China, No. 2292; Beijing, China, 2015.
Hermo, M. P.; Nemutlu, E.; Kir, S.; Barrón, D.; Barbosa, J. Improved determination of quinolones in milk at their MRL levels using LC-UV, LC-FD, LC-MS and LC-MS/MS and validation in line with regulation 2002/657/EC. Anal. Chim. Acta 2008, 613, 98–107.
Hermo, M. P.; Barrón, D.; Barbosa, J. Development of analytical methods for multiresidue determination of quinolones in pig muscle samples by liquid chromatography with ultraviolet detection, liquid chromatography-mass spectrometry and liquid chromagraphy-tandem mass spectrometry. J. Chromatogr. A 2006, 1104, 132–139.
Mirzajani, R.; Kardani, F. Fabrication of ciprofloxacin molecular imprinted polymer coating on a stainless steel wire as a selective solid-phase microextraction fiber for sensitive determination of fluoroquinolones in biological fluids and tablet formulation using HPLC-UV detection. J. Pharm. Biomed. Anal. 2016, 122, 98–109.
Piatkowska, M.; Jedziniak, P.; Zmudzki, J. Multiresidue method for the simultaneous determination of veterinary medicinal products, feed additives and illegal dyes in eggs using liquid chromatography-tandem mass spectrometry. Food Chem. 2016, 197, 571–580.
Wang, Z. H.; Zhu, Y.; Ding, S. Y.; He, F. Y.; Beier, R. C.; Li, J. C.; Jiang, H. Y.; Feng, C. W.; Wan, Y. P.; Zhang, S. X. et al. Development of a monoclonal antibody-based broad-specificity elisa for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal. Chem. 2007, 79, 4471–4483.
Peng, J.; Kong, D. Z.; Liu, L. Q.; Song, S. S.; Kuang, H.; Xu, C. L. Determination of quinoxaline antibiotics in fish feed by enzyme-linked immunosorbent assay using a monoclonal antibody. Anal. Methods 2015, 7, 5204–5209.
Liu, B.-H.; Chu, K.-C.; Yu, F.-Y. Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2016, 66, 1–7.
Cai, M. A.; Li, F.; Zhang, Y.; Wang, Q. B. One-pot polymerase chain reaction with gold nanoparticles for rapid and ultrasensitive DNA detection. Nano Res. 2010, 3, 557–563.
Curnis, F.; Fiocchi, M.; Sacchi, A.; Gori, A.; Gasparri, A.; Corti, A. NGR-tagged nano-gold: A new CD13-selective carrier for cytokine delivery to tumors. Nano Res. 2016, 9, 1393–1408.
Panfilova, E.; Shirokov, A.; Khlebtsov, B.; Matora, L.; Khlebtsov, N. Multiplexed dot immunoassay using Ag nanocubes, Au/Ag alloy nanoparticles, and Au/Ag nanocages. Nano Res. 2012, 5, 124–134.
Huang, P.; Tu, D. T.; Zheng, W.; Zhou, S. Y.; Chen, Z.; Chen, X. Y. Inorganic lanthanide nanoprobes for backgroundfree luminescent bioassays. Sci. China Mater. 2015, 58, 156–177.
Gu, Y.; Meng, G. W.; Wang, M. L.; Huang, Q.; Zhu, C. H.; Huang, Z. L. R6G/8-AQ co-functionalized Fe3O4@SiO2 nanoparticles for fluorescence detection of trace Hg2+ and Zn2+ in aqueous solution. Sci. China Mater. 2015, 58, 550–558.
Li, X.; Song, J.; Chen, B. L.; Wang, B.; Li, R.; Jiang, H. M.; Liu, J. F.; Li, C. Z. A label-free colorimetric assay for detection of c-Myc mRNA based on peptide nucleic acid and silver nanoparticles. Sci. Bull. 2016, 61, 276–281.
Ma, M.; Chen, H. R.; Shi, J. L. Construction of smart inorganic nanoparticle-based ultrasound contrast agents and their biomedical applications. Sci. Bull. 2015, 60, 1170–1183.
Lin, B. B.; Su, H. Y.; Jin, R. R.; Li, D. Y.; Wu, C. Q.; Jiang, X.; Xia, C. C.; Gong, Q. Y.; Song, B.; Ai, H. Multifunctional dextran micelles as drug delivery carriers and magnetic resonance imaging probes. Sci. Bull. 2015, 60, 1272–1280.
Li, J. X.; Zhao, Y. L. Nanotechnology in the programmed cell therapy: Nowhere to escape of cancer. Sci. Bull. 2016, 61, 45–47.
Li, F. H.; Bao, Y.; Wang, D. D.; Wang, W.; Niu, L. Smartphones for sensing. Sci. Bull. 2016, 61, 190–201.
Fu, C. H.; He, C. F.; Tan, L. F.; Wang, S. H.; Shang, L.; Li, L. L.; Meng, X. W.; Liu, H. Y. High-yield preparation of robust gold nanoshells on silica nanorattles with good biocompatiblity. Sci. Bull. 2016, 61, 282–291.
Xing, C. R.; Liu, L. Q.; Song, S. S.; Feng, M.; Kuang, H.; Xu, C. L. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453.
Rivas, L.; de la Escosura-Muñiz, A.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Triple lines gold nanoparticle-based lateral flow assay for enhanced and simultaneous detection of Leishmania DNA and endogenous control. Nano Res. 2015, 8, 3704–3714.
Wang, W. B.; Liu, L. Q.; Song, S. S.; Xu, L. G.; Kuang, H.; Zhu, J. P.; Xu, C. L. Gold nanoparticle-based strip sensor for multiple detection of twelve salmonella strains with a genus-specific lipopolysaccharide antibody. Sci. China Mater., in press, DOI: 10.1007/s40843-016-5077-0.
Lee, H.-J.; Ryu, Y.-J.; Tutkun, L.; Park, E.-K. Determination of oxolinic acid residues in the muscle tissue of olive flounder (Paralichthysolivaceus) by a lateral flow immunoassay. Food Agric. Immunol. 2016, 27, 367–376.
Zhi, A.-M.; Li, B.-B.; Liu, Q.-T.; Hu, X.-F.; Zhao, D.; Hou, Y.-Z.; Deng, R.-G.; Chai, S.-J.; Zhang, G.-P. Development of a lateral-flow immunochromatographic test device for the rapid detection of difloxacin residues. Food Agric. Immunol. 2010, 21, 335–345.
Zhao, Y. L.; Zhang, G. P.; Liu, Q. T.; Teng, M.; Yang, J. F.; Wang, J. H. Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of enrofloxacin residues. J. Agric. Food Chem. 2008, 56, 12138–12142.
Tochi, B. N.; Peng, J.; Song, S. S.; Liu, L. Q.; Kuang, H.; Xu, C. L. Determination of sarafloxacin and its analogues in milk using an enzyme-linked immunosorbent assay based on a monoclonal antibody. Anal. Methods 2016, 8, 1626–1636.
Li, S.; Xu, L. G.; Ma, W.; Kuang, H.; Wang, L. B.; Xu, C. L. Triple raman label-encoded gold nanoparticle trimers for simultaneous heavy metal ion detection. Small 2015, 11, 3435–3439.
Liu, L. Q.; Luo, L. J.; Suryoprabowo, S.; Peng, J.; Kuang, H.; Xu, C. L. Development of an immunochromatographic strip test for rapid detection of ciprofloxacin in milk samples. Sensors 2014, 14, 16785–16798.
Kong, D. Z.; Liu, L. Q.; Song, S. S.; Suryoprabowo, S.; Li, A. K.; Kuang, H.; Wang, L. B.; Xu, C. L. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 2016, 8, 5245–5253.
Wang, Z. H.; Zhang, H. Y.; Ni, H. J.; Zhang, S. X.; Shen, J. Z. Development of a highly sensitive and specific immunoassay for enrofloxacin based on heterologous coating haptens. Anal. Chim. Acta 2014, 820, 152–158.
Liu, N.; Zhao, Z. Y.; Tan, Y. L.; Lu, L.; Wang, L.; Liao, Y. C.; Beloglazova, N.; De Saeger, S.; Zheng, X. D.; Wu, A. B. Simultaneous raising of rabbit monoclonal antibodies to fluoroquinolones with diverse recognition functionalities via single mixture immunization. Anal. Chem. 2016, 88, 1246–1252.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (Nos. 21522102, 21503095, 21471068, 21371081, and 21301073), the Key Programs from MOST (Nos. 2016YFD0401101 and 2012YQ09019410), and grants from Natural Science Foundation of Jiangsu Province, MOF and MOE (Nos. BK20150145, BX20151038, BK20140003, BE2014672, BE2013613, BE2013611).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, J., Liu, L., Xu, L. et al. Gold nanoparticle-based paper sensor for ultrasensitive and multiple detection of 32 (fluoro)quinolones by one monoclonal antibody. Nano Res. 10, 108–120 (2017). https://doi.org/10.1007/s12274-016-1270-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1270-z