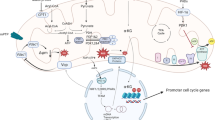
Abstract
The functional role of TGFβ type I receptor, activin-like kinase (ALK)-1 in post-myocardial infarction (MI) cardiac remodeling is unknown. We hypothesize that reduced ALK1 activity reduces survival and promotes cardiac fibrosis after MI. MI was induced in wild-type (WT), and ALK+/− mice by left coronary ligation. After 14 days ALK1+/− mice had reduced survival with a higher rate of cardiac rupture compared to WT mice. ALK1+/− left ventricles (LVs) had increased volumes at the end of systole and at the end of diastole. After MI ALK1+/− LVs had increased profibrotic SMAD3 signaling, type 1 collagen, and fibrosis as well as increased levels of TGFβ1 co-receptor, endoglin, VEGF, and ALK1 ligands BMP9 and BMP10. ALK1+/− LVs had decreased levels of stromal-derived factor 1α. These data identify the critical role of ALK1 in post-MI survival and cardiac remodeling and implicate ALK1 as a potential therapeutic target to improve survival after MI.
Graphical abstract





Similar content being viewed by others
Abbreviations
- MI:
-
Myocardial infarction
- TGFβ:
-
Transforming growth factor beta
- ALK1:
-
Activin-like kinase 1
- BMP:
-
Bone morphogenetic protein
- LCA:
-
Left coronary artery
- α-SMA:
-
α-smooth muscle actin
References
Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: the Framingham study. Am J Cardiol. 1979;44:53–9.
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596.
Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–81.
Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32.
Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549–61.
Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. CardiovascRes. 2007;74:184–95.
Bujak M, Ren G, Kweon HJ, et al. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–38.
Matsui Y, Morimoto J, Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J Biol Chem. 2010;1:69–80.
Lamouille S, Mallet C, Feige J-J, et al. Activin receptor–like kinase 1 is implicated in the maturation phase of angiogenesis. Blood. 2002;100:4495–501.
Oh SP, Seki T, Goss KA, et al. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–31.
Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–95.
Leask A. Getting to the heart of the matter. Circ Res. 2015;116:1269–76.
Morine KJ, Qiao X, Paruchuri V, et al. Reduced activin receptor-like kinase 1 activity promotes cardiac fibrosis in heart failure. CardiovascPathol. 2017;31:26–33.
Morine KJ, Qiao X, York S, et al. Bone morphogenetic protein 9 reduces cardiac fibrosis and improves cardiac function in heart failure. Circulation. 2018;138:513–26.
Fernández B, Durán AC, Fernández MC, et al. The coronary arteries of the C57BL/6 mouse strains: implications for comparison with mutant models. J Anat. 2008;212:12–8.
Scalise RFM, De Sarro R, Caracciolo A, et al. Fibrosis after myocardial infarction: an overview on cellular processes, molecular pathways, clinical evaluation and prognostic value. Med Sci. 2021;9:16.
Bhave S, Esposito M, Swain L, et al. Loss of bone morphogenetic protein-9 reduces survival and increases MMP activity after myocardial infarction. JACC Basic Transl Sci. 2023;8:1318–30.
Sullivan KE, Quinn KP, Tang KM, et al. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther. 2014;5:14.
David L, Mallet C, Mazerbourg S, et al. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–61.
Scharpfenecker M, Floot B, Russell NS, et al. The TGF-Î2 co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. RadiotherOncol. 2012;105:313–20.
Wu X, Reboll MR, Korf-Klingebiel M, et al. Angiogenesis after acute myocardial infarction. Cardiovasc Res. 2021;117:1257–73.
Sánchez-Elsner T, Botella LM, Velasco B, et al. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-β pathways*. J Biol Chem. 2002;277:43799–808.
Shao ES, Lin L, Yao Y, et al. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–206.
McDonald J, Bayrak-Toydemir P, DeMille D, et al. Curaçao diagnostic criteria for hereditary hemorrhagic telangiectasia is highly predictive of a pathogenic variant in ENG or ACVRL1 (HHT1 and HHT2). Genet Med. 2020;22:1201–5.
Hu X, Dai S, Wu W-J, et al. Stromal cell–derived factor-1α confers protection against myocardial ischemia/reperfusion injury. Circulation. 2007;116:654–63.
Saxena A, Fish JE, White MD, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31.
Hiasa K, Ishibashi M, Ohtani K, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61.
Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8.
Young K, Conley B, Romero D, et al. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120:4263–73.
Funding
This work was funded by the National Institute of Health grants R01HL133215 (NKK) and R01HL139785 (NKK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
Dr. Kapur receives institutional grant support and speaker/consulting honoraria from Abbott, Abiomed, Boston Scientific, LivaNova, Medtronic, MD Start, and Precardia.
Human and Animal Rights and Informed Consent
No human studies were carried out for the generation of data in this article.
Clinical Relevance
Post myocardial infarction wound healing process is crucial for the patient’s survival as well as the development of heart disease. The ALK1/BMP9 pathway is an emerging target for the development of novel therapies for both cancers and cardiovascular diseases. Data presented here sheds more light on the role of this pathway in the cardiac wound healing process thus identifying unique molecular targets for future treatments.
Additional information
Associate Editor Joost Sluijter oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 96 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhave, S., Swain, L., Qiao, X. et al. ALK1 Deficiency Impairs the Wound-Healing Process and Increases Mortality in Murine Model of Myocardial Infarction. J. of Cardiovasc. Trans. Res. (2023). https://doi.org/10.1007/s12265-023-10471-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-023-10471-w