Abstract
Exercise has been recognized as an important non-pharmacological approach for the prevention, treatment, and rehabilitation of cardiovascular diseases, but the mechanisms of exercise in promoting cardiovascular health remain unclear. Exercise generates cardiac benefits via stimulating muscle to secret hundreds of myokines that directly enter circulation and target heart tissue. Therefore, inter-organ communication between skeletal muscle and heart may be one important regulating pattern, and such communication can occur through secretion of molecules, frequently known as myokines. Irisin, a newly identified myokine, is cleaved from fibronectin type III domain-containing protein 5 (FNDC5) and secreted by the stimulation of exercise. Recently, accumulating evidence focusing on the interaction between irisin and cardiac function has been reported. This review highlights the molecular signaling by which irisin regulates the benefits of exercise on cardiac function both in physiological and pathological process, and discusses the clinical potential of irisin in treating heart diseases.
Graphical abstract
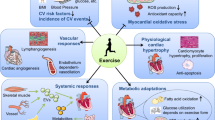
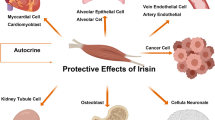
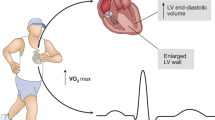
Exercise generates various cardiovascular benefits through stimulating skeletal muscle to secrete irisin. The exercise “hormone” irisin, both produced by exercise or recombinant form, exerts therapeutic effects in a group of cardiovascular disorders including heart failure, myocardial infarction, atherosclerosis and hypertension. However, the molecular mechanisms involved remain ambiguous.This review highlights the most up-to-date findings to bridge the gap between exercise, irisin and cardiovascular diseases, and discusses the potential clinical prospect of irisin



Similar content being viewed by others
References
Pinto FJ, Piñeiro D, Banerjee A, Perel P, Pervan B, Eiselé J-L. World Heart Day 2021: COVID-19, digital health, and tackling cardiovascular disease. Lancet. 2021;398(10310):1467–8. https://doi.org/10.1016/S0140-6736(21)02144-9.
Bloom D, Cafiero E, Jané-Llopis E, Abrahams-Gessel S, Bloom L, Fathima S, et al. The global economic burden of noncommunicable diseases. Geneva: World Economic Forum; 2011. https://www3.weforum.org/docs/WEF_Harvard_HE_GlobalEconomicBurdenNonCommunicableDiseases_2011.pdf. Accessed 21 May 2021.
Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. https://doi.org/10.1016/S0140-6736(04)17018-9.
Gelchu T, Abdela J. Drug therapy problems among patients with cardiovascular disease admitted to the medical ward and had a follow-up at the ambulatory clinic of Hiwot Fana Specialized University Hospital: the case of a tertiary hospital in eastern Ethiopia. SAGE Open Med. 2019;7:2050312119860401. https://doi.org/10.1177/2050312119860401.
Rubin DA, Nieminski KE, Monteferrante JC, Magee T, Reed GE, Herman MV. Ventricular arrhythmias after coronary artery bypass graft surgery: incidence, risk factors and long-term prognosis. J Am Coll Cardiol. 1985;6(2):307–10. https://doi.org/10.1016/S0735-1097(85)80165-0.
Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, et al. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12–85. https://doi.org/10.1093/eurheartj/ehz859.
Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6):S379–99. https://doi.org/10.1097/00005768-200106001-00007.
Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl Iii HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281(4):327–34.
Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–10.
Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M-C, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53. https://doi.org/10.1016/S0140-6736(11)60749-6.
Ferrer-Martínez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224(2):154–67. https://doi.org/10.1002/dvdy.10099.
Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297(1–2):79–83. https://doi.org/10.1016/S0378-1119(02)00828-4.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. https://doi.org/10.1038/nature10777.
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725–38. https://doi.org/10.1016/j.metabol.2012.09.002.
Huerta-Delgado AS, Roffe-Vazquez DN, Gonzalez-Gil AM, Villarreal-Calderón JR, Tamez-Rivera O, Rodriguez-Gutierrez NA, et al. Serum irisin levels, endothelial dysfunction, and inflammation in pediatric patients with type 2 diabetes mellitus and metabolic syndrome. J Diabetes Res. 2020;2020:1949415. https://doi.org/10.1155/2020/1949415.
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015;22(4):734–40. https://doi.org/10.1016/j.cmet.2015.08.001.
Yin C, Hu W, Wang M, Lv W, Jia T, Xiao Y. Irisin as a mediator between obesity and vascular inflammation in Chinese children and adolescents. Nutr Metab Cardiovasc Dis. 2020;30(2):320–9. https://doi.org/10.1016/j.numecd.2019.09.025.
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281(3):739–49. https://doi.org/10.1111/febs.12619.
Abedpoor N, Taghian F, Ghaedi K, Niktab I, Safaeinejad Z, Rabiee F, et al. PPARγ/Pgc-1α-Fndc5 pathway up-regulation in gastrocnemius and heart muscle of exercised, branched chain amino acid diet fed mice. Nutr Metab. 2018;15(1):59. https://doi.org/10.1186/s12986-018-0298-3.
Tsuchiya Y, Ando D, Takamatsu K, Goto K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. 2015;64(9):1042–50. https://doi.org/10.1016/j.metabol.2015.05.010.
Choi Y-K, Kim M-K, Bae KH, Seo H-A, Jeong J-Y, Lee W-K, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100(1):96–101. https://doi.org/10.1016/j.diabres.2013.01.007.
Yan B, Shi X, Zhang H, Pan L, Ma Z, Liu S, et al. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS One. 2014;9(4):e94235. https://doi.org/10.1371/journal.pone.0094235.
Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol. 2015;83(3):339–43. https://doi.org/10.1111/cen.12658.
Deng J, Zhang N, Chen F, Yang C, Ning H, Xiao C, et al. Irisin ameliorates high glucose-induced cardiomyocytes injury via AMPK/mTOR signal pathway. Cell Biol Int. 2020;44(11):2315–25. https://doi.org/10.1002/cbin.11441.
Guo Q, Wei X, Hu H, Yang D, Zhang B, Fan X, et al. The saturated fatty acid palmitate induces insulin resistance through Smad3-mediated down-regulation of FNDC5 in myotubes. Biochem Biophys Res Commun. 2019;520(3):619–26. https://doi.org/10.1016/j.bbrc.2019.10.077.
Seo DY, Bae JH, Kim TN, Kwak H-B, Kha PT, Han J. Exercise-induced circulating irisin level is correlated with improved cardiac function in rats. Int J Environ Res Public Health. 2020;17(11). https://doi.org/10.3390/ijerph17113863.
Patel CN. The signs and symptoms of coronary heart disease. East Afr Med J. 1963;40:319–21.
Bösner S, Becker A, Hani MA, Keller H, Sönnichsen AC, Haasenritter J, et al. Accuracy of symptoms and signs for coronary heart disease assessed in primary care. Br J Gen Pract. 2010;60(575):e246. https://doi.org/10.3399/bjgp10X502137.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update. Circulation. 2016;133(4):e38–360. https://doi.org/10.1161/CIR.0000000000000350.
Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. https://doi.org/10.1016/S0140-6736(16)31678-6.
Authors/Task Force M, Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J. 2012;33(20):2569–619. https://doi.org/10.1093/eurheartj/ehs215.
Murray CJL, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. https://doi.org/10.1016/S0140-6736(15)61340-X.
Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–62. https://doi.org/10.1016/j.neuroscience.2013.02.050.
Gür FM, Timurkaan S, Yalcin MH, Girgin A, Gençer TB. Immunohistochemical localization of irisin in mole rats (Spalax leucodon). Biotech Histochem. 2017;92(4):245–51. https://doi.org/10.1080/10520295.2017.1303194.
Sundarrajan L, Yeung C, Hahn L, Weber LP, Unniappan S. Irisin regulates cardiac physiology in zebrafish. PLoS One. 2017;12(8):e0181461. https://doi.org/10.1371/journal.pone.0181461.
Zhao YT, Wang J, Yano N, Zhang LX, Wang H, Zhang S, et al. Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart. J Cell Physiol. 2019;234(2):1671–81. https://doi.org/10.1002/jcp.27037.
Emanuele E, Minoretti P, Pareja-Galeano H, Sanchis-Gomar F, Garatachea N, Lucia A. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am J Med. 2014;127(9):888–90. https://doi.org/10.1016/j.amjmed.2014.04.025.
Anastasilakis AD, Koulaxis D, Kefala N, Polyzos SA, Upadhyay J, Pagkalidou E, et al. Circulating irisin levels are lower in patients with either stable coronary artery disease (CAD) or myocardial infarction (MI) versus healthy controls, whereas follistatin and activin A levels are higher and can discriminate MI from CAD with similar to CK-MB accuracy. Metabolism. 2017;73:1–8. https://doi.org/10.1016/j.metabol.2017.05.002.
Zuurbier CJ, Bertrand L, Beauloye CR, Andreadou I, Ruiz-Meana M, Jespersen NR, et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J Cell Mol Med. 2020;24(11):5937–54. https://doi.org/10.1111/jcmm.15180.
Xin T, Lu C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction. Aging. 2020;12(5):4474–88. https://doi.org/10.18632/aging.102899.
Liao Q, Qu S, Tang L-x, Li L-p, He D-f, Zeng C-y, et al. Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis. Acta Pharmacol Sin. 2019;40(10):1314–21. https://doi.org/10.1038/s41401-019-0230-z.
Zhao YT, Wang H, Zhang S, Du J, Zhuang S, Zhao TC. Irisin ameliorates hypoxia/reoxygenation-induced injury through modulation of histone deacetylase 4. PLoS ONE. 2016;11(11):e0166182. https://doi.org/10.1371/journal.pone.0166182.
Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998;1366(1):53–67. https://doi.org/10.1016/S0005-2728(98)00120-0.
Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation: mechanisms involved in H2O2 signaling. Antioxid Redox Signal. 2010;14(3):459–68. https://doi.org/10.1089/ars.2010.3363.
Li H, Qin S, Liang Q, Xi Y, Bo W, Cai M, et al. Exercise training enhances myocardial mitophagy and improves cardiac function via irisin/FNDC5-PINK1/parkin pathway in MI mice. Biomedicines. 2021;9(6). https://doi.org/10.3390/biomedicines9060701.
Chen Y-R, Chen C-L, Pfeiffer DR, Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282(45):32640–54. https://doi.org/10.1074/jbc.M702294200.
Davidson SM, Adameová A, Barile L, Cabrera-Fuentes HA, Lazou A, Pagliaro P, et al. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J Cell Mol Med. 2020;24(7):3795–806. https://doi.org/10.1111/jcmm.15127.
Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy. Circ Res. 2012;111(9):1208–21. https://doi.org/10.1161/CIRCRESAHA.112.265819.
Baechler BL, Bloemberg D, Quadrilatero J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy. 2019;15(9):1606–19. https://doi.org/10.1080/15548627.2019.1591672.
Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171(8):1890–906. https://doi.org/10.1111/bph.12516.
Vásquez-Trincado C, García-Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594(3):509–25. https://doi.org/10.1113/JP271301.
Narendra D, Tanaka A, Suen D-F, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. https://doi.org/10.1083/jcb.200809125.
Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584(7):1374–8. https://doi.org/10.1016/j.febslet.2010.02.017.
Tanida I, Ueno T, Kominami E. LC3 and autophagy. In: Deretic V, editor. Methods in molecular biology. Totowa: Humana Press; 2008. pp. 77–88.
Lu L, Ma J, Tang J, Liu Y, Zheng Q, Chen S, et al. Irisin attenuates myocardial ischemia/reperfusion-induced cardiac dysfunction by regulating ER-mitochondria interaction through a mitochondrial ubiquitin ligase-dependent mechanism. Clin Transl Med. 2020;10(5):e166. https://doi.org/10.1002/ctm2.166.
Metra M, Teerlink JR. Heart failure. Lancet. 2017;390(10106):1981–95. https://doi.org/10.1016/S0140-6736(17)31071-1.
Li R-L, Wu S-S, Wu Y, Wang X-X, Chen H-Y, Xin J-j, et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J Mol Cell Cardiol. 2018;121:242–55. https://doi.org/10.1016/j.yjmcc.2018.07.250.
Yu Q, Kou W, Xu X, Zhou S, Luan P, Xu X, et al. FNDC5/irisin inhibits pathological cardiac hypertrophy. Clin Sci. 2019;133(5):611–27. https://doi.org/10.1042/CS20190016.
Chen R-R, Fan X-H, Chen G, Zeng G-W, Xue Y-G, Liu X-T, et al. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGFβ1/Smad2/3 signaling axis. Chem Biol Interact. 2019;302:11–21. https://doi.org/10.1016/j.cbi.2019.01.031.
Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. https://doi.org/10.1152/ajpheart.00554.2011.
Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem. 2008;317(1):43–50. https://doi.org/10.1007/s11010-008-9803-8.
Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280(1):C53–60. https://doi.org/10.1152/ajpcell.2001.280.1.C53.
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13(5):619–24. https://doi.org/10.1038/nm1574.
Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: actions beyond metabolic regulation. J Mol Cell Cardiol. 2016;91:188–200. https://doi.org/10.1016/j.yjmcc.2016.01.001.
Horman S, Beauloye C, Vanoverschelde J-L, Bertrand L. AMP-activated protein kinase in the control of cardiac metabolism and remodeling. Curr Heart Fail Rep. 2012;9(3):164–73. https://doi.org/10.1007/s11897-012-0102-z.
Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122(3):489–505. https://doi.org/10.1161/CIRCRESAHA.117.311147.
Aronow WS, Fleg JL, Rich MW. Tresch and Aronow’s cardiovascular disease in the elderly. 6th ed. Boca Raton: CRC Press; 2019.
Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease. Circulation. 2007;115(4):450–8. https://doi.org/10.1161/CIRCULATIONAHA.106.637793.
The Emerging Risk Factors C. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–23. https://doi.org/10.1001/jama.2009.1063.
Davies MJ, Gordon JL, Gearing AJH, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171(3):223–9. https://doi.org/10.1002/path.1711710311.
O’Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Investig. 1993;92(2):945–51.
Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144(5):952.
Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, et al. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11(6):e0158038. https://doi.org/10.1371/journal.pone.0158038.
Lu J, Xiang G, Liu M, Mei W, Xiang L, Dong J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis. 2015;243(2):438–48. https://doi.org/10.1016/j.atherosclerosis.2015.10.020.
Zhang Y, Song H, Zhang Y, Wu F, Mu Q, Jiang M, et al. Irisin inhibits atherosclerosis by promoting endothelial proliferation through microRNA126-5p. J Am Heart Assoc. 2016;5(9):e004031. https://doi.org/10.1161/JAHA.116.004031.
Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. https://doi.org/10.1016/S0140-6736(18)32225-6.
Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157(4):527–36. https://doi.org/10.1111/j.1476-5381.2009.00240.x.
Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29(7):367–74. https://doi.org/10.1016/j.tips.2008.05.003.
Hermann M, Flammer A, Lüscher TF. Nitric oxide in hypertension. J Clin Hypertens. 2006;8(s12):17–29. https://doi.org/10.1111/j.1524-6175.2006.06032.x.
Panza JA. Endothelial dysfunction in essential hypertension. Clin Cardiol. 1997;20(S2):II26–33. https://doi.org/10.1002/j.1932-8737.1997.tb00009.x.
Inoue K, Fujie S, Hasegawa N, Horii N, Uchida M, Iemitsu K, et al. Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl Physiol Nutr Metab. 2019;45(7):715–22. https://doi.org/10.1139/apnm-2019-0602.
Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou L, et al. Irisin lowers blood pressure by improvement of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the spontaneously hypertensive rat. J Am Heart Assoc. 2016;5(11):e003433. https://doi.org/10.1161/JAHA.116.003433.
Liu C, Ngai C-Y, Huang Y, Ko W-H, Wu M, He G-W, et al. Depletion of intracellular Ca2+ stores enhances flow-induced vascular dilatation in rat small mesenteric artery. Br J Pharmacol. 2006;147(5):506–15. https://doi.org/10.1038/sj.bjp.0706639.
Goldsmith DJA, Covic AA, Venning MC, Ackrill P. Blood pressure reduction after parathyroidectomy for secondary hyperparathyroidism: further evidence implicating calcium homeostasis in blood pressure regulation. Am J Kidney Dis. 1996;27(6):819–25. https://doi.org/10.1016/S0272-6386(96)90519-3.
Du J, Wang X, Li J, Guo J, Liu L, Yan D, et al. Increasing TRPV4 expression restores flow-induced dilation impaired in mesenteric arteries with aging. Sci Rep. 2016;6(1):22780. https://doi.org/10.1038/srep22780.
Zhang P, Sun C, Li H, Tang C, Kan H, Yang Z, et al. TRPV4 (transient receptor potential vanilloid 4) mediates endothelium-dependent contractions in the aortas of hypertensive mice. Hypertension. 2018;71(1):134–42. https://doi.org/10.1161/HYPERTENSIONAHA.117.09767.
Ye L, Xu M, Hu M, Zhang H, Tan X, Li Q, et al. TRPV4 is involved in irisin-induced endothelium-dependent vasodilation. Biochem Biophys Res Commun. 2018;495(1):41–5. https://doi.org/10.1016/j.bbrc.2017.10.160.
Zhang W, Chang L, Zhang C, Zhang R, Li Z, Chai B, et al. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther. 2015;29(2):121–7. https://doi.org/10.1007/s10557-015-6580-y.
Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114(4):529–39. https://doi.org/10.1093/cvr/cvy023.
Tostes RCA, Wilde DW, Bendhack LM, Webb RC. Calcium handling by vascular myocytes in hypertension. Braz J Med Biol Res. 1997;30:315–23.
Jiang M, Wan F, Wang F, Wu Q. Irisin relaxes mouse mesenteric arteries through endothelium-dependent and endothelium-independent mechanisms. Biochem Biophys Res Commun. 2015;468(4):832–6. https://doi.org/10.1016/j.bbrc.2015.11.040.
Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56(3):351–8. https://doi.org/10.1161/HYPERTENSIONAHA.110.155663.
Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66(2):153–64. https://doi.org/10.1097/01.psy.0000116719.95524.e2.
Grassi G. Sympathetic neural activity in hypertension and related diseases. Am J Hypertens. 2010;23(10):1052–60. https://doi.org/10.1038/ajh.2010.154.
Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577(2):261–7. https://doi.org/10.1016/0006-8993(92)90282-E.
Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25(6):461–3. https://doi.org/10.1111/j.1440-1681.1998.tb02235.x.
Kang Y-M, He R-L, Yang L-M, Qin D-N, Guggilam A, Elks C, et al. Brain tumour necrosis factor-α modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83(4):737–46. https://doi.org/10.1093/cvr/cvp160.
Qi J, Zhang D-M, Suo Y-P, Song X-A, Yu X-J, Elks C, et al. Renin–angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol. 2013;13(1):48–54. https://doi.org/10.1007/s12012-012-9184-9.
Huo C-J, Yu X-J, Sun Y-J, Li H-B, Su Q, Bai J, et al. Irisin lowers blood pressure by activating the Nrf2 signaling pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Toxicol Appl Pharmacol. 2020;394:114953. https://doi.org/10.1016/j.taap.2020.114953.
Murry Charles E, Reinecke H, Pabon LM. Regeneration gaps. J Am Coll Cardiol. 2006;47(9):1777–85. https://doi.org/10.1016/j.jacc.2006.02.002.
Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68(6):1560–8. https://doi.org/10.1161/01.RES.68.6.1560.
Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72(1):19–44. https://doi.org/10.1146/annurev.physiol.010908.163111.
Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188. https://doi.org/10.1126/science.1077857.
Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35. https://doi.org/10.1038/nature10147.
Xie C, Zhang Y, Tran TDN, Wang H, Li S, George EV, et al. Irisin controls growth, intracellular Ca2+ signals, and mitochondrial thermogenesis in cardiomyoblasts. PLoS One. 2015;10(8):e0136816. https://doi.org/10.1371/journal.pone.0136816.
Deng J, Zhang N, Wang Y, Yang C, Wang Y, Xin C, et al. FNDC5/irisin improves the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction. Stem Cell Res Ther. 2020;11(1):228. https://doi.org/10.1186/s13287-020-01746-z.
Cao H, Kang BJ, Lee CA, Shung KK, Hsiai TK. Electrical and mechanical strategies to enable cardiac repair and regeneration. IEEE Rev Biomed Eng. 2015;8:114–24. https://doi.org/10.1109/RBME.2015.2431681.
Hassaan PS, Nassar SZ, Issa Y, Zahran N. Irisin vs. treadmill exercise in post myocardial infarction cardiac rehabilitation in rats. Arch Med Res. 2019;50(2):44–54. https://doi.org/10.1016/j.arcmed.2019.05.009.
Bashar SM, Samir El-sherbeiny SM, Boraie MZ. Correlation between the blood level of irisin and the severity of acute myocardial infarction in exercise-trained rats. J Basic Clin Physiol Pharmacol. 2019;30(1):59–71. https://doi.org/10.1515/jbcpp-2018-0090.
Han F, Zhang S, Hou N, Wang D, Sun X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309(9):H1501–8. https://doi.org/10.1152/ajpheart.00443.2015.
Barrett-Connor E. Diabetes and heart disease. Diabetes Care. 2003;26(10):2947–58. https://doi.org/10.2337/diacare.26.10.2947.
Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7(3):e256. https://doi.org/10.1038/nutd.2017.3.
Shirai K. Obesity as the core of the metabolic syndrome and the management of coronary heart disease. Curr Med Res Opin. 2004;20(3):295–304. https://doi.org/10.1185/030079903125003008.
Lopez-Legarrea P, de la Iglesia R, Crujeiras AB, Pardo M, Casanueva FF, Zulet MA, et al. Higher baseline irisin concentrations are associated with greater reductions in glycemia and insulinemia after weight loss in obese subjects. Nutr Diabetes. 2014;4(2):e110. https://doi.org/10.1038/nutd.2014.7.
Lin C, Guo Y, Xia Y, Li C, Xu X, Qi T, et al. FNDC5/irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin αV/β5-AKT signaling and reduction of oxidative/nitrosative stress. J Mol Cell Cardiol. 2021;160:27–41. https://doi.org/10.1016/j.yjmcc.2021.06.013.
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42. https://doi.org/10.1007/s11883-017-0678-6.
Wang Z, Chen K, Han Y, Zhu H, Zhou X, Tan T, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72(6):259–69. https://doi.org/10.1097/FJC.0000000000000608.
Wu F, Li Z, Cai M, Xi Y, Xu Z, Zhang Z, et al. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/irisin signaling pathway. Free Radic Biol Med. 2020;158:171–80. https://doi.org/10.1016/j.freeradbiomed.2020.06.038.
Mazur-Bialy AI, Kozlowska K, Pochec E, Bilski J, Brzozowski T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J Physiol Pharmacol. 2018;69(1):117–25. https://doi.org/10.26402/jpp.2018.1.13.
Tsuchiya Y, Ando D, Goto K, Kiuchi M, Yamakita M, Koyama K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J Exp Med. 2014;233(2):135–40. https://doi.org/10.1620/tjem.233.135.
Jandova T, Buendía-Romero A, Polanska H, Hola V, Rihova M, Vetrovsky T, et al. Long-term effect of exercise on irisin blood levels—systematic review and meta-analysis. Healthcare. 2021;9(11). https://doi.org/10.3390/healthcare9111438.
Huang J, Wang S, Xu F, Wang D, Yin H, Lai Q, et al. Exercise training with dietary restriction enhances circulating irisin level associated with increasing endothelial progenitor cell number in obese adults: an intervention study. PeerJ. 2017;5:e3669. https://doi.org/10.7717/peerj.3669.
Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12(4):274–7. https://doi.org/10.1016/S0899-9007(96)00000-8.
Brasier AR. The nuclear factor-κB–interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86(2):211–8. https://doi.org/10.1093/cvr/cvq076.
Deng X, Huang W, Peng J, Zhu T-T, Sun X-L, Zhou X-Y, et al. Irisin alleviates advanced glycation end products-induced inflammation and endothelial dysfunction via inhibiting ROS-NLRP3 inflammasome signaling. Inflammation. 2018;41(1):260–75. https://doi.org/10.1007/s10753-017-0685-3.
Erickson HP. Irisin and FNDC5 in retrospect. Adipocyte. 2013;2(4):289–93. https://doi.org/10.4161/adip.26082.
Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell. 2018;175(7):1756-68.e17. https://doi.org/10.1016/j.cell.2018.10.025.
Funding
This study was funded in part by the Natural Science Foundation of Jiangsu Province (BK20201435).
Author information
Authors and Affiliations
Contributions
All authors agreed to the study conception. Material preparation, data collection, and analysis were performed by Bin Wang, Chen Zhao, and Yuanxin Wang. The first draft of the manuscript was written by Baishu Zhu and revised by Yalan Zhou, Junjie Lin, and Renqing Zhao. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study does not contain any human subjects or animals performed by any of the authors.
Informed Consent
This study does not include any human participants performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, B., Wang, B., Zhao, C. et al. Irisin Regulates Cardiac Responses to Exercise in Health and Diseases: a Narrative Review. J. of Cardiovasc. Trans. Res. 16, 430–442 (2023). https://doi.org/10.1007/s12265-022-10310-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10310-4