Abstract
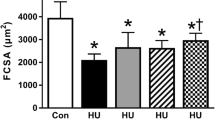
We investigated the inhibitory effect of active hexose correlated compound (AHCC) on muscle atrophy in hindlimb-suspended rats. Twenty-four six-week-old male Sprague-Dawley rats were randomly divided into three groups: the control sedentary group (CS, n = 8), the hindlimb-suspended group (HS, n = 8), and the hindlimb-suspended and AHCC-supplemented group (HSA, n = 8). Hindlimb suspension and AHCC supplementation were performed for two weeks. The HSA group was treated with AHCC (1 g/1 kg of body weight (BW)) orally in 0.3 mL of PBS solution, while the HS group received the vehicle (PBS solution) only. After two weeks, the cross-sectional area (CSA) of the HS and HSA groups decreased by approximately 36% (p < 0.05) and 19%, respectively, compared to the CS group. In addition, myonuclear numbers of the HS and HSA groups and the extensor digitorum longus (EDL) muscle weight of the HS group decreased 30% (p < 0.05) and 18%, respectively, compared to the CS group. AHCC supplementation increased the phosphorylation of pAkt/Akt in the HSA group compared to the HS group (p < 0.05). Furthermore, Fbx32 and MuRF1 protein expression in the HSA group recovered to the level of the CS group. Based on these results, AHCC supplementation may have a positive role in the prevention of muscle atrophy via Akt activation in hindlimb-suspended rats.
Similar content being viewed by others
References
Bodine, S. C. and L. M. Baehr (2014) Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 307: E469–484.
Waters, D. L. and R. N. Baumgartner (2011) Sarcopenia and obesity. Clin. Geriatr. Med. 27: 401–42.
Dogan, M., B. Karadag, T. Ozyigit, S. Kayaoglu, A. Ozturk, Y. Altuntas (2012) Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta Clinica Belgica 67: 328–332.
Baek, S., G. Nam, K. Han, S. Choi, S. Jung, A. Bok, Y. Kim, K. Lee, B. Han, and D. Kim (2014) Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008–2010 Korea National Health and Nutrition Examination Survey. J. Endocrinol. Invest. 37: 247–260.
Abbatecola, A. M., G. Paolisso, P. Fattoretti, W. J. Evans, V. Fiore, L. Dicioccio, and F. Lattanzio (2011) Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J. Nutr. Health Aging 15: 890–895.
Sacheck, J. M., J. P. Hyatt, A. Raffaello, R. T. Jagoe, R. R. Roy, V. R. Edgerton, S. H. Lecker, and A. L. Goldberg (2007) Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 21: 140–155.
Peng, X. D., P. Z. Xu, M. L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W. S. Chen, S. E. Crawford, K. G. Coleman, and N. Hay (2003) Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes. Dev. 17: 1352–1365.
Stitt, T. N., D. Drujan, B. A. Clarke, F. Panaro, Y. Timofeyva, W. O. Kline, M. Gonzalez, G. D. Yancopoulos, and D. J. Glass (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 14: 395–403.
Bodine, S. C., E. Latres, S. Baumhueter, V. K. Lai, L. Nunez, B. A. Clarke, W. T. Poueymirou, F. J. Panaro, E. Na, K. Dharmarajan, Z. Q. Pan, D. M. Valenzuela, T. M. DeChiara, T. N. Stitt, G. D. Yancopoulos, and D. J. Glass (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708.
Gomes, M. D., S. H. Lecker, R. T. Jagoe, A. Navon, and A. L. Goldberg (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 98: 14440–14445.
Always, S. E., H. Degens, G. Krishnamurthy, and A. Chaudhrai (2003) Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J. Gerontol. A. Biol. Sci. Med. Sci. 58: B687–B697.
Always, S. E., J. K. Martyn, J. Ouyang, A. Chaudhrai, and Z. S. Murlasits (2003) Id2 expression during apoptosis and satellite cell activation in unloaded and loaded quail skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284: R540–549.
Borisov, A. B. and B. M. Carlson (2000) Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat. Rec. 258: 305–318.
Léger, B., R. Senese, A.W. Al-Khodairy, O. Dériaz, C. Gobelet, J. P. Giacobino, and A. P. Russell (2009) Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3β and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 40: 69–78.
Yoshimura, K. and K. Harii (1999) A regenerative change during muscle adaptation to denervation in rats. J. Surg. Res. 81: 139–146.
Adams, G. and F. Haddad (1996) The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J. Appl. Physiol. 81: 2509–2516.
Carson, J. A. (1997) The regulation of gene expression in hypertrophying skeletal muscle. Exerc. Sport Sci. Rev. 25: 301–320.
Galvan, E., E. Arentson-Lantz, S. Lamon, and D. Paddon-Jones (2016) Protecting skeletal muscle with protein and amino acid during periods of disuse. Nutrients 8: 404.
Gundersen, K. (2016) Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J. Exp. Biol. 219: 235–242.
Matsushita, K., Y. Kuramitsu, Y. Ohiro, M. Obara, M. Kobayashi, Y. Q. Li, and M. Hosokawa (1998) Combination therapy of active hexose correlated compound plus UFT significantly reduces the metastasis of rat mammary adenocarcinoma. Anticancer Drugs 9: 343–350.
Ghoneum, M., M. Wimbley, F. Salem, A. McKlain, N. Attallah, and G. Gill (1995) Immunomodulatory and anticancer effects of active hemicellulose compound (AHCC). Int. J. Immunother. 11: 23–28.
Kidd, P. M. (2000) The use of mushroom glucans and proteoglycans in cancer treatment. Altern. Med. Rev. 5: 4–27.
Borchers, A. T., J. S. Stern, R. M. Hackman, C. L. Keen, and M. E. Gershwin (1999) Mushrooms, tumors, and immunity. Proc. Soc. Exp. Biol. Med. 221: 281–293.
Mizuno, T., P. Yeohlui, T. Kinoshita, C. Zhuang, H. Ito, and Y. Mayuzumi (1996) Antitumor activity and chemical modification of polysaccharides from niohshimeji mushroom, Tricholma giganteum. Biosci. Biotechnol. Biochem. 60: 30–33.
Sakagami, H., T. Aoki, A. Simpson, and S. Tanuma (1991) Induction of immunopotentiation activity by a protein-bound polysaccharide, PSK (review). Anticancer Res. 11: 993–999.
Wasser, S. P. and A. L. Weis (1999) Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit. Rev. Immunol. 19: 65–96.
Segarra, S., G. Miro, A. Montoya, L. Pardo-Marin, J. Teichenné, L. Ferrer, and J. J. Cerón (2018) Prevention of disease progression in Leishmania infantum-infected dogs with dietary nucleotides and active hexose correlated compound. Parasit. Vectors 11: 103–112.
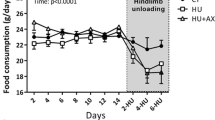
Aviles, H., T. Belay, K. Fountain, M. Vance, and B. Sun (2003) Sonnenfeld, G. Active hexose correlated compound enhances resistance to Klebsiella pneumoniae infection in mice in the hindlimb-unloading model of spaceflight conditions. J. Appl. Physiol. 95: 491–496.
Morey-Holton, E. R. and R. K. Globus (2002) Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 92: 1367–1377.
Bodine, S. C. and K. Baar (2012) Analysis of skeletal muscle hypertrophy in models of increased loading. Myogenesis 798: 213–229.
Baghirova, S., B. G. Hughes, M. J. Hendzel, and R. Schulz (2015) Sequential fractionation and isolation of subcellular proteins from tissue or cultured cells. MethodsX 2: 440–445.
Holeček, M. and S. Mičuda (2017) Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation. Physiol. Res. 66: 959–967.
Blaauw, B., C. Marta, L. Agatea, L. Toniolo, C. Mammucari, E. Masiero, R. Abraham, M. Sandri, S. Schiaffino, and C. Reggiani (2010) Inducible activation of akt increases skeletal muscle mass and force without satellite cell activation. Biophysical. J. 98: 153a.
Izumiya, Y., T. Hopkins, C. Morris, K. Sato, L. Zeng, J. Viereck, J. A. Hamilton, N. Ouchi, N. K. LeBrasseur, and K. Walsh (2008) Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 7: 159–172.
Dong, F., Y. Hua, P. Zhao, J. Ren, M. Du, and N. Sreejayan (2009) Chromium supplement inhibits skeletal muscle atrophy in hindlimb-suspended mice. J. Nutr. Biochem. 20: 992–999.
Matsui, K., T. Ozaki, M. Oishi, Y. Tanaka, M. Kaibori, M. Nishizawa, T. Okumura, and A. H. Kwon (2011) Active hexose correlated compound inhibits the expression of proinflammatory biomarker iNOS in hepatocytes. Eur. Surg. Res. 47: 274–283.
Kadi, F., P. Schjerling, L. L. Andersen, N. Charifi, J. L. Madsen, L. R. Christensen, and J. L. Andersen (2004) The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J. Physiol. 558: 1005–1012.
Lange, S., F. Xiang, A. Yakovenko, A. Vihola, P. Hackman, E. Rostkova, J. Kristensen, B. Brandmeier, G. Franzen, B. Hedberg, L. G. Gunnarsson, S. M. Hughes, S. Marchand, T. Sejersen, I. Richard, L. Edström, E. Ehler, B. Udd, and M. Gautel (2005) The kinase domain of titin controls muscle gene expression and protein turnover. Science 308: 1599–1603.
McElhinny, A. S., K. Kakinuma, H. Sorimachi, S. Labeit, and C. C. Gregorio (2002) Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J. Cell Biol. 157: 125–136.
Pizon, V., A. Iakovenko, P. F. Van Der Ven, R. Kelly, C. Fatu, D. O. Furst, E. Karsenti, and M. Gautel (2002) Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J. Cell Sci. 115: 4469–4482.
Oishi, Y., T. Ogata, K. I. Yamamoto, M. Terada, T. Ohira, Y. Ohira, K. Taniguchi, and R. R. Roy (2008) Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol. (Oxf). 192: 381–395.
Acknowledgments
The authors extend their thanks to the subjects whose participation made this study possible. This work was supported by the Soonchunhyang University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, YJ., Bae, SH., Park, JY. et al. AHCC Supplementation Attenuates Muscle Atrophy via Akt Activation in Hindlimb-suspended Rat. Biotechnol Bioproc E 24, 476–482 (2019). https://doi.org/10.1007/s12257-018-0482-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-018-0482-3