Abstract
Introduction
Many devices (e.g., nebulizers and spacers) are used to deliver aerosol in a non-invasive ventilation circuit (NIV) without any special recommendation. The aim of the present work was to compare the doses delivered from seven different aerosol delivery systems when placed in the NIV using automatic continuous positive airway pressure (Auto-CPAP).
Methods
Three spacers and three vibrating mesh nebulizers were compared to a Sidestream jet nebulizer (SIDE). Each device was placed proximal to a breathing simulator in a standard NIV circuit with a 500 ml tidal volume, 15 breaths/min and a 1:3 inspiratory-expiratory ratio. Two ml of salbutamol solution containing 10,000 μg was nebulized using Aerogen Pro (PRO), Aerogen Solo (SOLO), NIVO and SIDE. Twelve metered dose inhaler doses, containing 100 μg salbutamol each, were delivered using AeroChamber MV (AC), AeroChamber Vent (VC) and AeroChamber Mini (MC). Total emitted dose (TED) and its percentage were determined. Aerodynamic droplet characteristics were measured using cooled Andersen Cascade Impactor.
Results
The vibrating mesh nebulizers used had significantly more (p < 0.001) TED compared to the jet nebulizer. The spacers used had higher TED % (p < 0.001) compared to the nebulizers. The fine particle fraction of SIDE was the highest (p = 0.021) and mass median aerodynamic diameter of the spacers was the smallest (p = 0.001). The fine particle dose from vibrating mesh nebulizers was the greatest (p = 0.02).
Conclusions
Aerosol delivery in Auto-CPAP NIV is feasible; however, aerosol delivery method should be chosen or substituted with care. 2 mg delivered from a spacer would be equivalent to 3 mg nebulized from a vibrating mesh nebulizer and 5 mg nebulized from a Sidestream.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aerosols are commonly used with mechanically ventilated patients [1, 2]. They are delivered by a metered dose inhaler (MDI) with or without spacer modification to be used in ventilator circuits or by nebulizers [1, 2]. Previous studies using patients with stable asthma [3] and COPD [4] confirmed that aerosol delivery in bilevel positive airway pressure (BiPAP) non-invasive ventilation circuit (NIV) is feasible and can be effective. Many studies had compared the use of different nebulizers [2, 4–9] and different spacers [10–15] in the BiPAP NIV circuit. However, few studies have compared different nebulizers and MDI with spacers in the same study or the practicability of aerosol delivery in a continuous positive airway pressure (CPAP) NIV circuit. CPAP applies a pre-set pressure level on a continuous basis. CPAP devices apply continuous positive airway pressure throughout the breathing cycle. Thus, the ventilator itself does not cycle during CPAP, no additional pressure above the level of CPAP is provided, and patients initiate all of their breaths. On the other hand, an Automatic Continuous Positive Airway Pressure ventilator (Auto-CPAP) automatically titrates the amount of pressure delivered to the patient on a breath-by-breath basis by measuring the resistance in the subject’s breathing, changing its level over time [16, 17]. Some ventilators include more modalities of ventilation including CPAP and Auto-CPAP.
Additionally, jet nebulizers should ideally be replaced by inhalers which do not distribute aerosol into the environment, e.g., MDI spacers or valve holding chambers when treating patients with, for example, Middle East respiratory syndrome [18].
The European Respiratory Society Guidelines on the use of nebulizers [19] recommend the determination of the aerodynamic characteristics of the droplets in the emitted dose. However, it had been reported that the Comité-Européen-Normalisation (2001) (CEN) method to measure the aerodynamic characteristics, proposed by the Guidelines [19], should not be used due to the effects of evaporation. Instead the use of a cooled Next Generation Impactor is recommended [20]. The Anderson Cascade Impactor (ACI) was also validated to measure the aerodynamic characterization of the dose delivered by nebulizers [21]. Only the CEN method that identifies the fate of the nebulized dose using sinus flow breath simulation, and filters to entrain the inhalation and exhalation output of a nebulized, dose should be used.
The aim of the present work was to identify equivalent doses for COPD Auto-CPAP NIV patients due to the reported differences in output and performance between different nebulizers [20, 22] and different spacers [1, 23]. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Methods
Delivery Systems
A 10,000 μg (in 2 ml) salbutamol respiratory solution (Farcolin respirator solution, 5000 μg ml−1; Pharco Pharmaceuticals, Alexandria, Egypt) was nebulised using the Aerogen® Pro [PRO] Nebuliser and Aerogen Solo [SOLO] Nebuliser (Aerogen Limited, Galway, Ireland), NIVO™ Nebuliser (Aerogen/Philips, Andover, MA, USA) and the Sidestream [SIDE] nebuliser (Philips, Andover, MA, USA) attached to a PortaNeb compressor (Philips Respironics, UK). The PortaNeb compressor provides an air flow of 6 l min−1 into the nebuliser to aerosolize the liquid. Twelve MDI doses containing 100 μg salbutamol (Ventoline, GlaxoSmithKline, Cairo, Egypt) each were delivered using AeroChamber® MV [AC], AeroChamber Vent [VC] and AeroChamber Mini [MC] spacers (Trudell Medical International, London, Canada).
The choice of salbutamol dosage for different devices was in accordance with the previous literatures [1, 20, 22–25].
In-Vitro Fate of the Aerosolized Dose Using an Automatic Continuous Positive Airway Pressure (Auto-CPAP) System Used for NIV
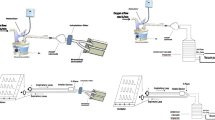
Each of the seven aerosol delivery methods (previously described) were assembled according to Fig. 1 which was designed to mimic that of a patient receiving NIV [5].
A breathing simulation machine (model 5600i, Grand Rapids, USA) was connected to an Auto-CPAP (3B Medical, Lake Wales, USA) (Fig. 1). The NIV breathing circuit consisted of a 180 cm length of corrugated tubing (diameter of 22 mm) and a fixed leak expiration port (B&D Electromedical, Warwickshire, UK). Spontaneous breathing was simulated to provide a tidal volume of 500 ml with a rate of 15 breaths per minute and inspiratory to expiratory phase ratio of 1:3. The breathing simulator setting was chosen to mimic the breathing pattern of COPD patients when non-invasive support ventilation was prescribed during acute exacerbations at local hospitals. The outlet of each nebulizer was attached to its standard T-piece and both outlets were connected into the NIV circuit tubing with a tight seal. In addition, each spacer was placed into the NIV circuit and both outlets were connected to the NIV circuit tubing with a tight seal.
An electrostatic filter pad enclosed in a filter holder (Pari GmbH, Starnberg, Germany) was attached next to the breathing machine (inhalation filter). This filter would entrain all the aerosol produced during the inhalation period of a breathing cycle and thus provides a good measure of the total inhaled aerosol dose (the in vitro total emitted dose available for inhalation). Another electrostatic filter was attached next to the Auto-CPAP (ventilator filter) to check if any aerosol reaches the ventilator. A third electrostatic filter was placed 4 cm above the outlet of the expiration port of the NIV system (expiration filter). A vacuum of 25 l min−1 was drawn through this filter to ensure that it captured the entire dose that was expelled out of the NIV system [5].
The nebulizer position within the ventilator circuit was proximal to the breathing simulator with the expiration port positioned after it towards the ventilator [5]. The Auto-CPAP was switched on until it reached a pressure of 7 cm H2O (about 10 min), then the breathing machine was switched on 30 s before delivering the aerosol. Salbutamol solution, 10,000 µg in 2 ml, was nebulized to sputtering for the SIDE and to dryness for PRO, SOLO and NIVO. Twelve MDI doses containing 100 μg salbutamol each were delivered using AC, VC and MC. Each dose of the 12 MDI doses was actuated at the start of the inspiratory phase of the breathing cycle.
For each aerosol delivery method five determinations were made (n = 5). The amounts of salbutamol entrained on each filter, left in the nebulizer chamber and deposited inside the tubing or the spacer used were recovered by rinsing with 90% acetonitrile. Amounts entrained on the filter were sonicated with 90% acetonitrile prior to rinsing. High-performance liquid chromatography (HPLC) with UV detection was used to identify amounts of salbutamol. The method used a 25 mm × 4.6 mm ZORBAX Eclipse Plus C18, ODS1 column (Agilant, Santa Clara, USA) through which a mobile phase consisted of a mixture of acetonitrile and water [containing 0.1% phosphoric acid] (90:10, v/v) was pumped at 1 ml min−1 using Agilent 1260 Infinity preparative pump (G1361A), Agilent 1260 Infinity Diode array detector VL (G131SD) was set at 225 nm with an injection volume 100 µl. Calibration solutions ranged from 4 to 100 μg ml−1 (w/v). The limit of detection was 0.35 µg ml−1 and the lower limit of quantification was 2.55 µg ml−1.
The Aerodynamic Particle Size Characterization Using the ACI
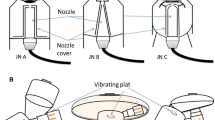
An Anderson Cascade Impactor [ACI] (Copley Scientific Ltd, Nottingham, UK) was used to determine the particle droplet size distribution of the aerosolized drug that would be delivered to the patient. The experimental set-up is described in Fig. 2. This Figure shows that the ACI was always proximal to the breathing simulator to collect and measure aerosol that was going to the inhalation filter. The position of each aerosol delivery method was proximal to the ACI.
The ACI, with its plates in situ, was placed in a refrigerator at 5 °C for 60 min before use [21]. Hence, the induction port of the ACI was connected directly into the NIV circuit with an air-tight seal. The vacuum flow through the ACI apparatus was provided by a vacuum pump (Brook Crompton, Huddersfield, UK). The flow rate was measured using an electronic digital flow meter (MKS Instruments, Andover, USA).
For each aerosol delivery method three determinations were made (n = 3). The time to start the Auto-CPAP and breathing machine and the doses delivered by the nebulizers and the spacers as described in the previous section.
Salbutamol deposited on each plate of the ACI, nebulization chamber, spacer and tubing was recovered by rinsing with 90% acetonitrile. Similarly, the mass entrained on the filters was recovered by sonication and rinsing. HPLC used as described in the previous section.
The fine particle dose (FPD), fine particle fraction (FPF %) and the mass median aerodynamic diameter (MMAD) was determined using Copley Inhaler Testing Data Analysis Software (CITDAS, Copley Scientific, Nottingham, UK) impactor data analysis software.
Statistical Analysis
All data are expressed as mean (SD). One-way analysis of variance (ANOVA) with the application of least significant difference correction was used to compare the seven different aerosol delivery methods with SPSS V17.0 (SPSS Inc., Chicago, USA).
Results
Table 1 provides a summary of the fate of the delivered dose. No salbutamol was recovered from the ventilation filter or the tubing between this filter and the expiration port. No salbutamol was recovered from the expiration port filter when using any of the spacers to deliver the inhaled dose. Statistical analysis revealed that there was a significant (p < 0.001) difference in the amounts recovered on the inhalation filters (Total emitted dose) between the seven methods. Similarly there were significant differences (p < 0.001) for the residual volumes and the amount deposited in the T-piece and the amount deposited on the spacer. For the three vibrating mesh nebulizers used there was significantly more (p < 0.001) salbutamol entrained on the inhalation filter compared to the jet nebulizer. In addition, the amounts deposited on the tubing and expiration port were significantly greater (p < 0.001) for the three vibrating mesh nebulizers, but amounts left in the nebulizer was significantly greater (p < 0.001) for SIDE.
As shown in Table 1, we used percentage to compare the nebulizers to the spacers. The three spacers used had higher percentages of salbutamol deposited on the inhalation filter (p < 0.001) compared to the nebulizers. In addition, the percentages of salbutamol entrained in the spacer were higher than those in the nebulization chambers (p < 0.001). However, the percentages of salbutamol deposited on the tubing and the exhalation port filter were higher for the nebulizers (p < 0.001). No significant difference between the three vibrating mesh nebulizers as well as between all the three spacers in all parameters.
The aerodynamic droplet size distribution from each aerosol delivery method is shown in Fig. 3 with a summary in Table 2. Consistent with the above results no drug was recovered on the ventilation filter or deposited in the tubing of the NIV circuit between the expiration port and ventilator. In addition, no salbutamol was recovered from the expiration port filter when using any of the spacers to deliver the inhaled dose. The FPF of SIDE was the highest (p = 0.021), MMAD of the AC and MC spacers was the smallest (p = 0.001) and the FPD from the SOLO nebulizer was the greatest (p < 0.02).
The NIVO had relatively smaller FPF and FPD compared to the other two vibrating mesh nebulizers, but there was no significant difference.
All of the spacers used had a smaller MMAD (p = 0.001) and relatively higher FPF, but not significant, compared to the three vibrating mesh nebulizers used. The VC resulted in aerosol with relatively higher MMAD and lower FPF than the AC and the MC, but was not significant.
Discussion
Placing either the Aerogen Pro, as a vibrating mesh nebulizer, or the Sidestream, as a jet nebulizer between expiration port and breathing simulator was previously proven to produce a higher delivery of the drug to the inhalation filter with less lost through the expiration port [5, 7]. Hence, we placed the seven used inhalation devices in the above position. The Auto-CPAP added positive pressure which will direct air towards the patient through the inspiratory phase. Therefore, the entire aerosolized dose in the inspiratory phase is aimed at the inhalation filter. During expiratory phase more aerosols would exit from the expiration port. However, during an expiratory phase, Auto-CPAP keeps up positive pressure hence; patient’s airways do not collapse when they breathe out. This positive pressure allows exhaled air to leave the expiration port so that the patient inhales fresh air. However, the positive pressure is adequate to avoid the total escape of the dose nebulized during the expiratory phase. Hence, some aerosols are held within the NIV circuit [5, 7]. Deposition due to gravity in expiration phase is not significant because of the droplet small size and the very limited time for the expiratory phase, which would be 3 s as mentioned before in the methodology (1: 3 inspiratory to expiratory ration). When the inspiratory phase starts the aerosol kept in the NIV circuit, nebulized during the expiratory phase, was directed towards the breathing simulator (hence patient).
The holding effect of the nebulized aerosol within the NIV tubing in the expiratory phase was similar to that when an MDI was used with a spacer, hence might enhance evaporation and therefore decreases droplets size [5, 7].
The MMAD for the Aerogen Pro and Sidestream were 4.5 and 3.9 µm, respectively, which is comparable to our previous study using a BiPAP NIV circuit [5]. However, in an earlier study using the same method and nebulizer without the NIV, MMADs were 5.0 and 4.2 µm [20] respectively. In addition FPF in the NIV circuit was higher. Similar to other previous studies [26] these variations show that evaporation could really occur in the NIV circuit. This suggests that aerosol delivery to the lungs of NIV patients would be better than when a patient uses the conventional nebulization method [5]. In addition, it is advisable to remove the humidifier (which might increase the droplet size by condensation), that many patients using CPAP are utilizing, when a bronchodilator is administered [17].
No salbutamol was found on the ventilator filter or tubing between this filter and the expiration port. This was due to the 840 ml inner volume of the NIV tubing and the 500 ml tidal volume together with the Auto-CPAP positive pressure through the expiratory phase [5].
The results of the three tested vibrating mesh nebulizers were comparable in all the parameters. Although Sidestream MMAD was smaller and FPF was greater than all tested vibrating mesh nebulizers, there is likely little clinical significance because the difference is small, especially when compared to SOLO. However, an average of 1.5-fold greater FPD of vibrating mesh nebulizers, compared to the Sidestream, would supply greater lung deposition and likely be of clinical significance. This great difference is due to the smaller residual volume in all vibrating mesh nebulizers used [5, 27, 28]. Although pulmonary clinical response when using any of the tested inhalation methods might be at the top of the dose response relationship, the critical clinical effect would be from larger systemic absorption of the vibrating mesh nebulizers if we did not change the dose. These results suggest that approximately 2 mg nebulized from any of the tested vibrating mesh nebulizers would be equivalent to 3 mg nebulized from a Sidestream. This difference between vibrating mesh nebulizers and jet nebulizers in the Auto-CPAP NIV circuit is similar to those reported in the BiPAP NIV circuit [4, 5, 29, 30] and highlights the need to use a lower dose when switching a patient to a vibrating mesh nebulizer. These results also highlight the feasibility of aerosol delivery in the Auto-CPAP NIV circuit.
However, when using any of the tested vibrating mesh nebulizers the condensation of the aerosol on the tubing of the NIV was about threefold that of the jet nebulizer [5, 28]. This could result in wasting a high percentage of the nebulized dose in the tubing. This was due to the downward aerosolization of the vibrating mesh nebulizers compared to the upward aerosolization of the jet nebulizer which results in the return of any condensate to the jet nebulizer chamber [5, 28].
The results of the three tested spacers were comparable in all the parameters. The spacers used showed no significant deposition on the expiration port filter at all. This could be attributed to the release of all the MDI delivered doses in the spacers at the start of the inspiratory phase allowing the entire delivered dose to be directed to the patient [31]. The TED deposited on the inhalation filter of the spacers used was about one-fifth and one-third of that of the vibrating mesh nebulizer and the jet nebulizer, respectively. However, the nominal dose placed into the nebulizer was about seven times that placed in the spacer. This resulted in an average TED % from the spacer that was 1.5-fold that of the vibrating mesh nebulizers and 2.5-fold that of the jet nebulizer. These results suggest that approximately 2 mg delivered from a spacer would be equivalent to 3 mg nebulized from a vibrating mesh nebulizer and 5 mg nebulized from a Sidestream. Ari et al. [25] in the BiPAP NIV circuit; showed that the delivery efficiency with the MDI was threefold greater than with the jet nebulizer (p = 0.002).
In addition, about 50% of the delivered dose was deposited on the wall of the spacers tested. It is well known that particles deposited in the spacer have a very large aerodynamic particle size that would not reach the lung and could cause adverse effect. Hence, spacers could decrease the unwanted adverse effect when used to deliver aerosol in the NIV circuit compared to the nebulizer. In addition, all the spacers tested resulted in very small MMADs compared to all the nebulizers tested. This suggests that evaporation effects were more prominent due to the longer distance of the actuated dose in the spacer to the ACI, resulting in further evaporation. The large amount of drug with large particle size deposited on the wall of the spacer, the better TED % and the smaller MMAD from the spacers used could suggest better lung deposition with lower dose and lower side effect when using a spacer in a NIV.
A study by Power et al. [27] showed a superiority of the NIVO vibrating mesh nebulizer over the jet nebulizer and the MDI in the percentage of the aerosol delivered to the patient when placed in the NIV [27]. Power et al. did not state when they actuated the dose (Perhaps during the expiratory phase). In addition, they did not use a spacer which might improve their MDI results. An additional variable could be the types of salbutamol used in the study as it is known that different types of salbutamol have variable aerosol characteristics [32]. Hence, this may add some variation to the results provided from the MDI with spacers and nebulizers.
The seven tested inhalation device are often used in similar clinical conditions. However, according to our results, to deliver 2 mg (20 doses) efficiently by the spacer to the NIV patient, health care providers need to stand for more than 1 min (about 80 s), as they have to adjust the actuation of each dose at the start of the inspiratory phase. Hence, they need to be focused when delivering 2 mg by a spacer to result in a comparable lung deposition of 3 mg aerosolized by a vibrating mesh nebulizer or 5 mg by a jet nebulizer, which only requires a press of a button to start delivering the dose. Hence, using spacers might be considered time consuming to the health care provider. This, together with the significant differences in the aerodynamic properties of the delivered dose from the jet nebulizers, vibrating mesh nebulizers and spacers tested in a NIV circuit, highlights why preliminary in vitro data is required for all inhalation delivery methods before introduction in the NIV.
Conclusions
Aerosol delivery during Auto-CPAP NIV is practical and would be of clinical benefit to patients. Whilst the aerodynamic characteristics of the jet nebulizer were slightly more encouraging for lung deposition, there was a much larger FPD from the vibrating mesh nebulizers due to their smaller residual volume. The spacers had a higher TED %, lower MMAD compared to all the tested nebulizers. The in vitro results imply that 2 mg drug delivered from a spacer, which may be more time consuming for the health care provider, would be equivalent to 3 mg nebulized from a vibrating mesh nebulizer and 5 mg nebulized from a Sidestream jet nebulizer. The extent of this difference suggests that; although the proper dosage of salbutamol is often based on the desired clinical effect; the need to find out in vitro data for all inhalation delivery methods before substitution or introduction into the NIV circuit is also very important.
References
Berlinski A, Chavez A. Albuterol delivery via metered dose inhaler in a spontaneously breathing pediatric tracheostomy model. Pediatr Pulmonol. 2013;48(10):1026–34.
Ari A, Restrepo RD. Aerosol delivery device selection for spontaneously breathing patients: 2012. Respir Care. 2012;57(4):613–26.
Parkes SN, Bersten AD. Aerosol kinetics and bronchodilator efficacy during continuous positive airway pressure delivered by face mask. Thorax. 1997;52(2):171.
Abdelrahim M, Plant P, Chrystyn H. The relative lung and systemic bioavailability of terbutaline following nebulisation in non-invasively ventilated patients. Int J Pharm. 2011;420(2):313–8.
Abdelrahim ME, Plant P, Chrystyn H. In-vitro characterisation of the nebulised dose during non-invasive ventilation. J Pharm Pharmacol. 2010;62(8):966–72.
Carvalho TC, McCook JP, Narain NR, McConville JT. Development and characterization of phospholipid-stabilized submicron aqueous dispersions of coenzyme Q10 presenting continuous vibrating-mesh nebulization performance. J Liposome Res. 2013;23(4):276–90.
Dai B, Kang J, Sun L-F, Tan W, Zhao H-W. Influence of exhalation valve and nebulizer position on albuterol delivery during noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv. 2014;27(2):125–32.
Piastra M, De Luca D, De Carolis MP, Tempera A, Stival E, Caliandro F, et al. Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr Pulmonol. 2012;47(8):757–62.
Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Rosen Farrar J. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD J Chronic Obstr Pulm Dis. 2012;9(1):58–72.
Garner SS, Wiest DB, Stevens CE, Habib DM. Effect of Heliox on albuterol delivery by metered dose inhaler in pediatric in vitro models of mechanical ventilation. Pharmacother J Hum Pharmacol Drug Ther. 2006;26(10):1396–402.
Halliday HL, Patterson CC, Halahakoon CW. A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics. 2001;107(2):232–40.
Lugo RA, Keenan J, Salyer JW. Accumulation of CO2 in reservoir devices during simulated neonatal mechanical ventilation. Pediatr Pulmonol. 2000;30(6):470–5.
Garner SS, Wiest DB, Bradley JW. Albuterol delivery by metered-dose inhaler in a pediatric high-frequency oscillatory ventilation model. Crit Care Med. 2000;28(6):2086–9.
Wildhaber JH, Dore ND, Devadason SG, LeSouëf PN, Hayden MJ. Salbutamol delivery from a hydrofluoroalkane pressurized metered-dose inhaler in pediatric ventilator circuits an in vitro study. Chest J. 1998;113(1):186–91.
Mitchell JP, Nagel MW. In vitro performance testing of three small volume-holding chambers under conditions that correspond with use by infants and small children. J Aerosol Med. 1997;10(4):341–9.
Donn SM, Sinha SK. Invasive and noninvasive neonatal mechanical ventilation. Respir Care. 2003;48(4):426–41.
Donn SM, Sinha SK. 8.1 Conventional mechanical ventilation. Pediatric Neonatal Mech Vent. 2015;149–274.
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–94.
Boe J, Dennis J, O’Driscoll B, Members of Task Force, Bauer T, Carone M et al. European Respiratory Society Guidelines on the use of nebulizers: Guidelines prepared by a European Respiratory Society Task Force on the use of nebulizers. Eur Respir J. 2001;18(1):228–42.
Abdelrahim ME, Chrystyn H. Aerodynamic characteristics of nebulized terbutaline sulphate using the next generation impactor (NGI) and CEN method. J Aerosol Med Pulm Drug Deliv. 2009;22(1):19–28.
Abdelrahim ME. Aerodynamic characteristics of nebulized terbutaline sulphate using the Andersen Cascade Impactor compared to the Next Generation Impactor. Pharm Dev Technol. 2011;16(2):137–45.
Pallin M, Naughton MT. Noninvasive ventilation in acute asthma. J Crit Care. 2014;29(4):586–93.
DiBlasi RM, Coppolo DP, Nagel MW, Doyle CC, Avvakoumova VI, Ali RS, et al. A novel, versatile valved holding chamber for delivering inhaled medications to neonates and small children: laboratory simulation of delivery options. Respir Care. 2010;55(4):419–26.
Boukhettala N, Porée T, Diot P, Vecellio L. In vitro performance of spacers for aerosol delivery during adult mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2014;28(2):130–6.
Ari A, Harwood RJ, Sheard MM, Fink JB. Pressurized metered-dose inhalers versus nebulizers in the treatment of mechanically ventilated subjects with artificial airways: an in vitro study. Respir Care. 2015;60(11):1570–4.
Calvert L, Jackson J, White J, Barry P, Kinnear W, O’callaghan C. Enhanced delivery of nebulised salbutamol during non-invasive ventilation. J Pharm Pharmacol. 2006;58(11):1553–7.
Power P, MacLoughlin R, Wolny M, Duffy C. Evaluation of vibrating mesh nebulizer, jet nebulizer and pMDI performance during simulated adult and paediatric CPAP and NIPPV. J Aerosol Med Pulm Drug Del. 2013;26(2):A50–1.
Michotte J-B, Jossen E, Roeseler J, Liistro G, Reychler G. In vitro comparison of five nebulizers during noninvasive ventilation: analysis of inhaled and lost doses. J Aerosol Med Pulm Drug Deliv. 2014;27(6):430–40.
Dubus JC, Vecellio L, De Monte M, Fink JB, Grimbert D, Montharu J, et al. Aerosol deposition in neonatal ventilation. Pediatr Res. 2005;58(1):10.
Fink J, Uster P, Schmidt D, Simon M. In vitro characteristics of the aeroneb portable nebulizer system. J Aerosol Med. 2001;14(3):1–11.
Hussein RRS, M. A. Ali A, Salem HF, Abdelrahman MM, Said ASA, Abdelrahim MEA. In vitro/in vivo correlation and modeling of emitted dose and lung deposition of inhaled salbutamol from metered dose inhalers with different types of spacers in noninvasively ventilated patients. Pharm Dev Technol. 2015:1–10. doi:10.3109/10837450.2015.1116567. (In Press).
Smyth HD. The influence of formulation variables on the performance of alternative propellant-driven metered dose inhalers. Adv Drug Deliv Rev. 2003;55(7):807–28.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Ahmed Hassan, Hoda Rabea, Raghda R.S. Hussein: Experiment, data entry, writing. Maha M Abdelrahman: Analytical method development. Randa Salah Eldin, Amira S.A. Said, Heba F. Salem: Concept, study design. Mohamed E. Abdelrahim: Concept, planning of study design, statistics, and writing.
Disclosures
A. Hassan, H. Rabea, R. R. S. Hussein, R. S. Eldin, M. M. Abdelrahman, A. S. A. Said, H. F. Salem, M. E. Abdelrahim have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hassan, A., Rabea, H., Hussein, R.R.S. et al. In-Vitro Characterization of the Aerosolized Dose During Non-Invasive Automatic Continuous Positive Airway Pressure Ventilation. Pulm Ther 2, 115–126 (2016). https://doi.org/10.1007/s41030-015-0010-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-015-0010-y